25.8 Disaccharides
We saw in Section 25.6 that reaction of a monosaccharide with an alcohol yields a glycoside in which the anomeric –OH group is replaced by an –OR substituent. If the alcohol is itself a sugar, the glycosidic product is a disaccharide.
Maltose and Cellobiose
Disaccharides contain a glycosidic acetal bond between the anomeric carbon of one sugar and an –OH group at any position on the other sugar. A glycosidic bond between C1 of the first sugar and the –OH at C4 of the second sugar is particularly common. Such a bond is called a 1→4 link.
The glycosidic bond to an anomeric carbon can be either α or β. Maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two α-D-glucopyranose units joined by a 1→4-α-glycoside bond. Cellobiose, the disaccharide obtained by partial hydrolysis of cellulose, consists of two β-D-glucopyranose units joined by a 1→4-β– glycoside bond.
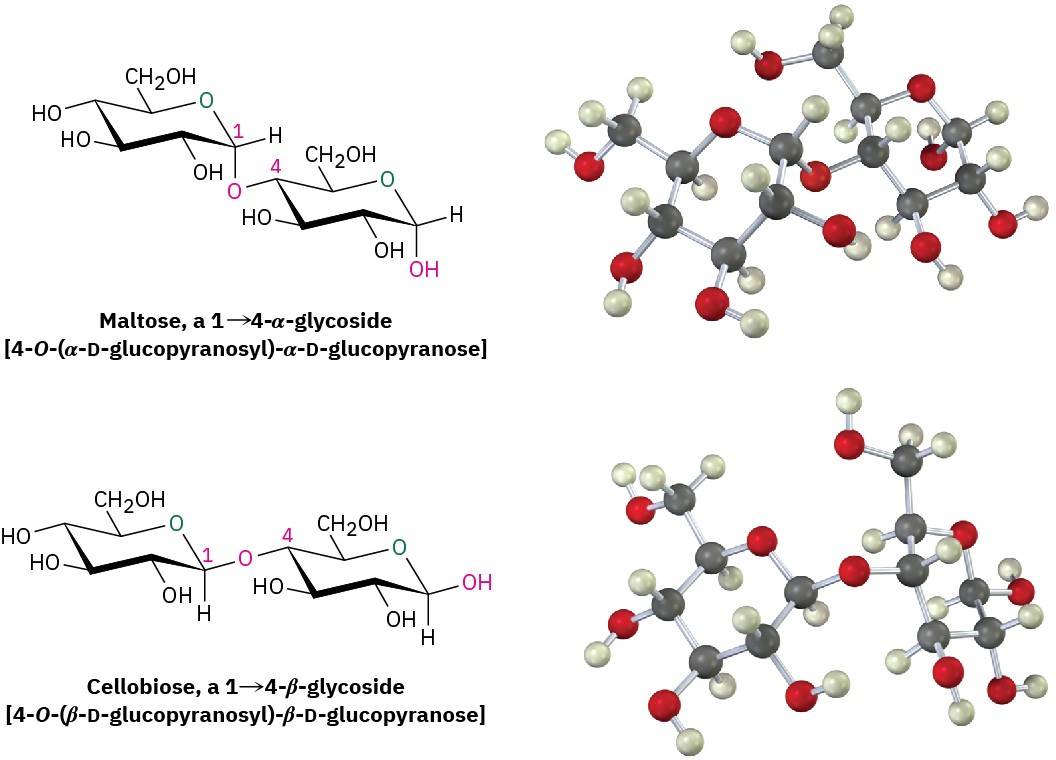
Maltose and cellobiose are both reducing sugars because the anomeric carbons on their right-hand glucopyranose units have hemiacetal groups and are in equilibrium with aldehyde forms. For a similar reason, both maltose and cellobiose exhibit mutarotation of α and β anomers of the glucopyranose unit on the right.
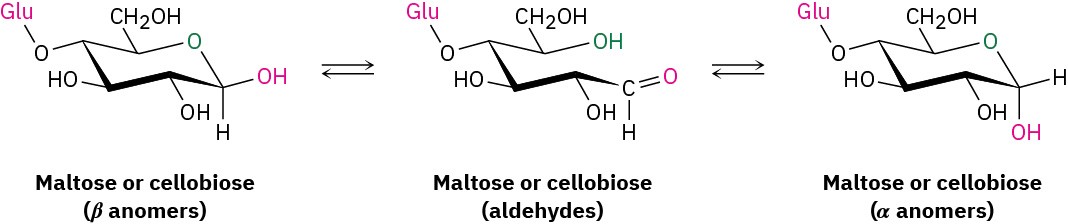
Despite the similarities of their structures, cellobiose and maltose have dramatically different biological properties. Cellobiose can’t be digested by humans and can’t be fermented by yeast. Maltose, however, is digested without difficulty and is fermented readily.
Problem 25-25
Show the product you would obtain from the reaction of cellobiose with the following reagents:
(a) NaBH4
(b)
Br2, H2O
(c)
CH3COCl, pyridine
Lactose
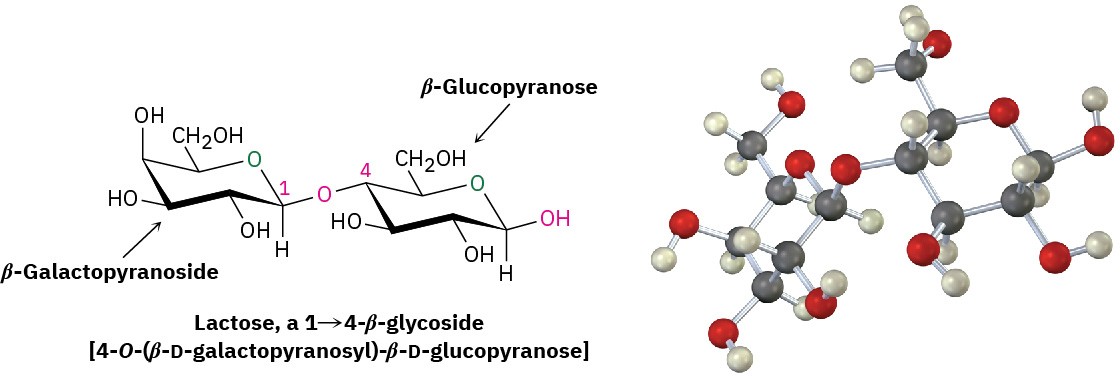
Lactose is a disaccharide that occurs naturally in both human and cow’s milk. It is widely used in baking and in commercial milk formulas for infants. Like maltose and cellobiose, lactose is a reducing sugar. It exhibits mutarotation and is a 1→4-β-linked glycoside. Unlike maltose and cellobiose, however, lactose contains two different monosaccharides—D- glucose and D-galactose—joined by a β-glycosidic bond between C1 of galactose and C4 of glucose.
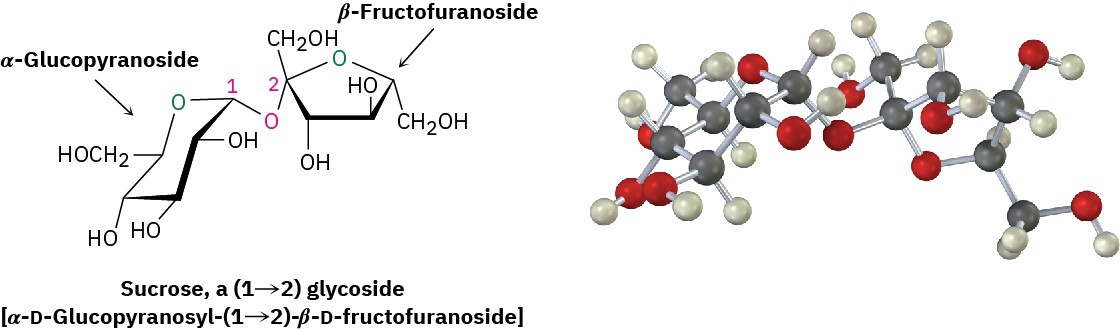
Sucrose
Sucrose, or ordinary table sugar, is probably the most abundant pure organic chemical in the world. Whether from sugar cane (20% sucrose by weight) or sugar beets (15% by weight), and whether raw or refined, all table sugar is sucrose.
Sucrose is a disaccharide that yields 1 equivalent of glucose and 1 equivalent of fructose on hydrolysis. This 1 : 1 mixture of glucose and fructose is often referred to as invert sugar because the sign of optical rotation changes, or inverts, during the hydrolysis of sucrose ([α]D = +66.5) to a glucose/ fructose mixture ([α]D = –22.0). Some insects, such as honeybees, have enzymes called invertases that catalyze the sucrose hydrolysis. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
Unlike most other disaccharides, sucrose is not a reducing sugar and does not undergo mutarotation. These observations imply that sucrose is not a hemiacetal and that glucose and fructose must both be glycosides. This can happen only if the two sugars are joined by a glycoside link between the anomeric carbons of both sugars—C1 of glucose and C2 of fructose.