Chemistry Matters — Chiral Drugs
The many hundreds of different pharmaceutical agents approved for use by the U.S. Food and Drug Administration come from many sources. Many drugs are isolated directly from plants or bacteria, and others are made by chemical modification of naturally occurring compounds. An estimated 33%, however, are made entirely in the laboratory and have no relatives in nature.

Figure 5.18 The S enantiomer of ibuprofen soothes the aches and pains of athletic injuries much more effectively than the R enantiomer. (credit: “World Athletic Championships 2007 in Osaka” by Eckhard Pecher/Wikimedia Commons, CC BY 2.5)
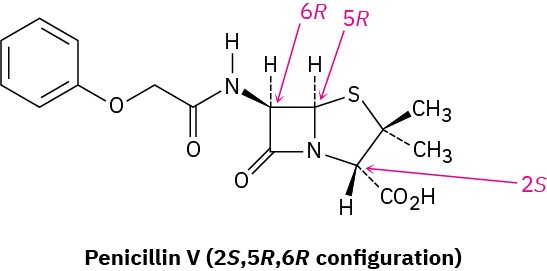
Those drugs that come from natural sources, either directly or after laboratory modification, are usually chiral and are generally found only as a single enantiomer rather than as a racemate. Penicillin V, for example, an antibiotic isolated from the Penicillium mold, has the 2S,5R,6R configuration. Its enantiomer, which does not occur naturally but can be made in the laboratory, has no antibiotic activity.
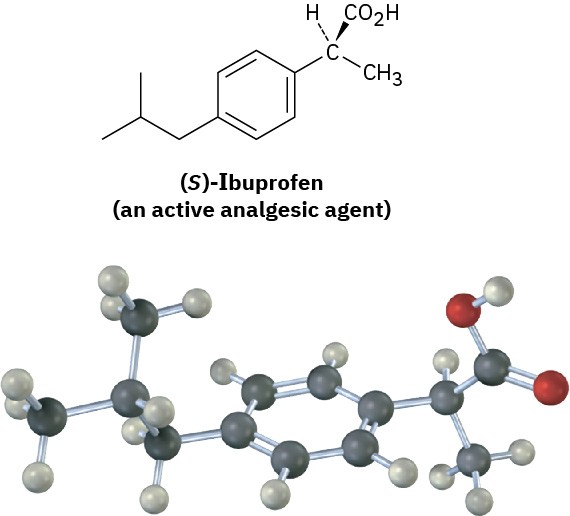
In contrast to drugs from natural sources, drugs that are made entirely in the laboratory are either achiral or, if chiral, are often produced and sold as racemates. Ibuprofen, for example, has one chirality center and is sold commercially under such trade names as Advil, Nuprin, and Motrin as a 50 : 50 mixture of R and S. It turns out, however, that only the
S enantiomer is active as an analgesic and anti-inflammatory agent. The R enantiomer of ibuprofen is inactive, although it is slowly converted in the body to the active S form.
Not only is it chemically wasteful to synthesize and administer an enantiomer that does not serve the intended purpose, many instances are now known where the presence of the “wrong” enantiomer in a racemic mixture either affects the body’s ability to utilize the “right” enantiomer or has unintended pharmacological effects of its own. The presence of (R)-ibuprofen in the racemic mixture, for instance, slows the rate at which the S enantiomer takes effect in the body, from 12 minutes to 38 minutes.
To get around this problem, pharmaceutical companies attempt to devise methods of enantioselective synthesis, which allow them to prepare only a single enantiomer rather than a racemic mixture. Viable methods have been developed for the preparation of (S)- ibuprofen, which is marketed in Europe. We’ll look further into enantioselective synthesis in the Chapter 19 Chemistry Matters.

