5.10 Chirality at Nitrogen, Phosphorus, and Sulfur
As noted previously, the most common cause of chirality in a molecule is the presence of four different substituents bonded to a tetrahedral atom. Although that atom is usually carbon, it doesn’t necessarily have to be. Nitrogen, phosphorus, and sulfur atoms are all commonly encountered in organic molecules, and can all be chirality centers. We know, for instance, that trivalent nitrogen is tetrahedral, with its lone pair of electrons acting as the fourth “substituent” (Section 1.10). Is trivalent nitrogen chiral? Does a compound such as ethylmethylamine exist as a pair of enantiomers?
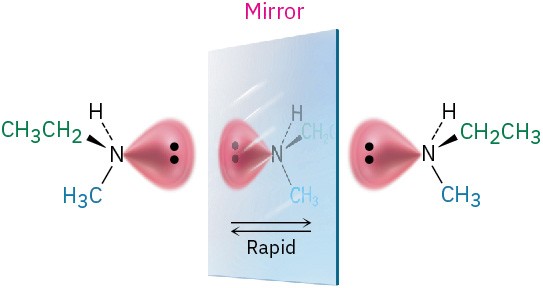
The answer is both yes and no. Yes in principle, but no in practice. It turns out that most trivalent nitrogen compounds undergo a rapid umbrella-like inversion that interconverts enantiomers, so we can’t isolate individual enantiomers except in special cases.
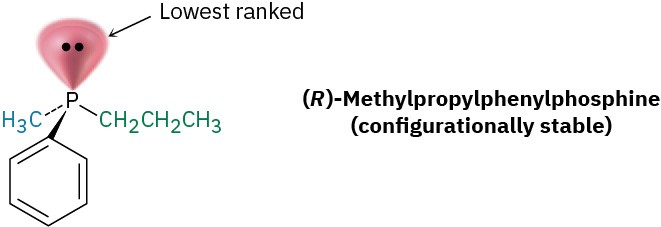
A similar situation occurs in trivalent phosphorus compounds, called phosphines, but the inversion at phosphorus is substantially slower than inversion at nitrogen, so stable chiral phosphines can be isolated. (R)- and (S)-methylpropylphenylphosphine, for instance, are configurationally stable for several hours at 100 °C. We’ll see the importance of phosphine chirality in Section 26.7 in connection with the synthesis of chiral amino acids.
Divalent sulfur compounds are achiral, but trivalent sulfur compounds called sulfonium salts (R3S+) can be chiral. Like phosphines, sulfonium salts undergo relatively slow inversion, so chiral sulfonium salts are configurationally stable and can be isolated.
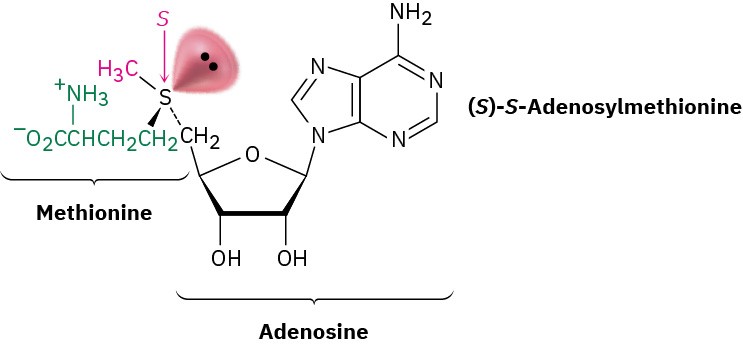
Perhaps the best known example is the coenzyme S-adenosylmethionine, the so-called biological methyl donor, which is involved in many metabolic pathways as a source of CH3 groups. (The “S” in the name S-adenosylmethionine stands for sulfur and means that the adenosyl group is attached to the sulfur atom of the amino acid methionine.) The molecule has S stereochemistry at sulfur and is configurationally stable for several days at room temperature. Its R enantiomer is also known but is not biologically active.