31.1 Chain-Growth Polymers
Synthetic polymers are classified by their method of synthesis as either chain-growth or step-growth. These categories are somewhat imprecise but nevertheless provide a useful distinction. Chain-growth polymers are produced by chain-reaction polymerization in which an initiator adds to the carbon–carbon double bond of an unsaturated substrate (a vinyl monomer) to yield a reactive intermediate. This intermediate reacts with a second molecule of monomer to yield a new intermediate, which reacts with a third monomer unit, and so on.
The initiator can be a radical, an acid, or a base. Historically, as we saw in Section 8.10, radical polymerization was the most common method because it can be carried out with practically any vinyl monomer.
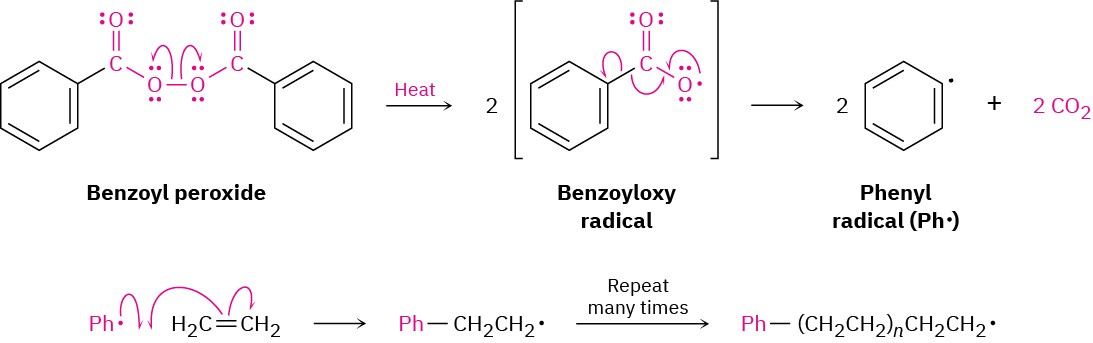
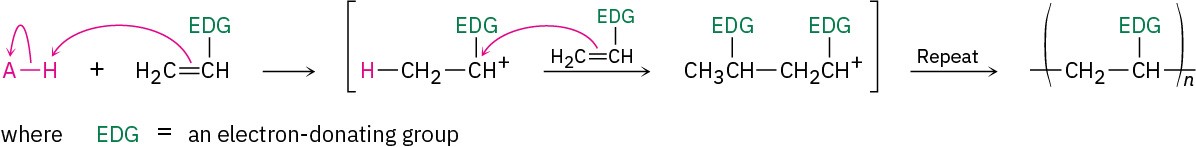
Acid-catalyzed (cationic) polymerization, by contrast, is effective only with vinyl monomers that contain an electron-donating group (EDG) capable of stabilizing the chain-carrying carbocation intermediate.
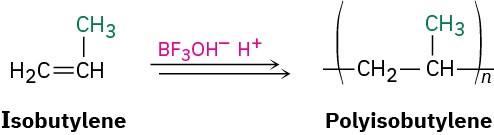
Isobutylene (2-methylpropene) is a good example of a monomer that polymerizes rapidly under cationic conditions. The reaction is carried out commercially at –80 °C, using BF3 and a small amount of water to generate BF3OH– H+ catalyst. The product is used in the manufacture of truck and bicycle inner tubes.
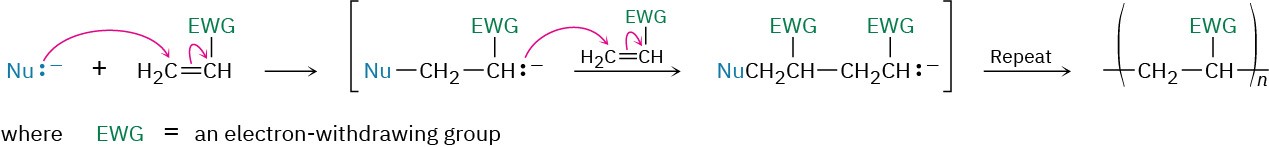
Vinyl monomers with electron-withdrawing groups (EWG) can be polymerized by basic (anionic) catalysts. The chain-carrying step is a conjugate nucleophilic addition of an anion to the unsaturated monomer (Section 19.13).
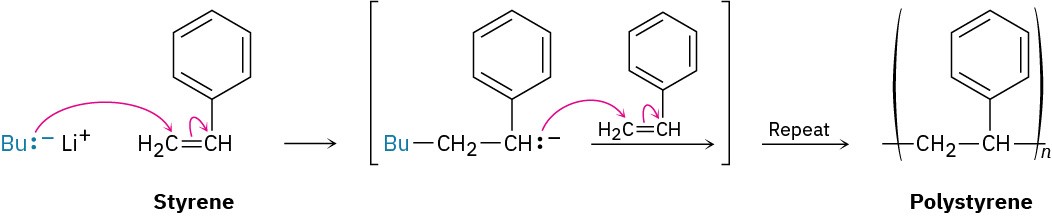
Acrylonitrile (H!C═CHCN), methyl methacrylate [H!C═C(CH“)CO!CH“], and styrene (H!C═CHC#H$) can all be polymerized anionically. The polystyrene used in foam coffee
cups, for example, is prepared by anionic polymerization of styrene using butyllithium as catalyst.
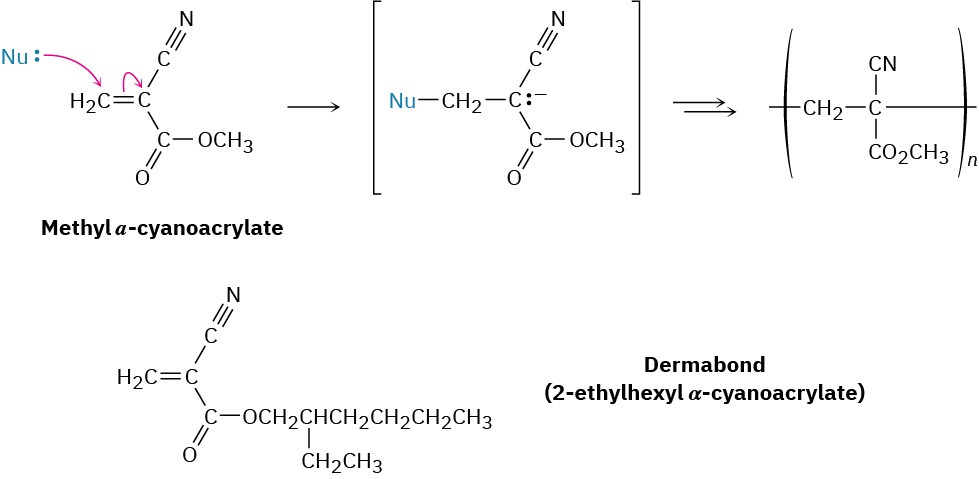
An interesting example of anionic polymerization accounts for the remarkable properties of “super glue,” one drop of which can support up to 2000 lb. Super glue is simply a solution of pure methyl α-cyanoacrylate, which has two electron-withdrawing groups that make anionic addition particularly easy. Trace amounts of water or bases on the surface of an object are sufficient to initiate polymerization of the cyanoacrylate and bind articles together. Skin is a good source of the necessary basic initiators, and many people have found their fingers stuck together after inadvertently touching super glue. So good is super glue at binding tissues that related cyanoacrylate esters such as Dermabond are often used in place of sutures to close wounds.
Problem 31-1
Order the following monomers with respect to their expected reactivity toward cationic polymerization, and explain your answer:
H2C═CHCH3, H2C═CHCl, H2C═CH–C6H5, H2C═CHCO2CH3
Problem 31-2
Order the following monomers with respect to their expected reactivity toward anionic polymerization, and explain your answer:
H2C═CHCH3, H2C═CHC≡N, H2C═CHC6H5
Problem 31-3
Polystyrene is produced commercially by reaction of styrene with butyllithium as an anionic initiator. Using resonance structures, explain how the chain-carrying intermediate is stabilized.

