17.9 Phenols and Their Uses
The outbreak of World War I provided a stimulus for the industrial preparation of large amounts of synthetic phenol, which was needed as a raw material to manufacture the explosive, picric acid (2,4,6-trinitrophenol). Today, approximately 132 million tons of phenol is manufactured worldwide each year for use in such products as Bakelite resin and adhesives for binding plywood.
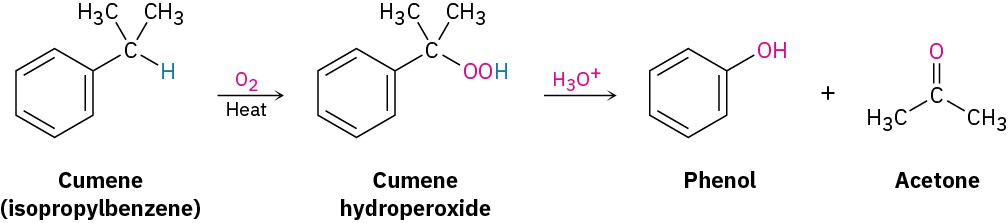
Phenol was manufactured for many years by the Dow process, in which chlorobenzene reacts with NaOH at high temperature and pressure (Section 16.7). Now, however, an alternative synthesis uses isopropylbenzene, commonly called cumene. Cumene reacts with air at high temperature by benzylic oxidation through a radical mechanism to form cumene hydroperoxide, which is converted into phenol and acetone by treatment with acid. This is a particularly efficient process because two valuable chemicals are prepared at the same time.
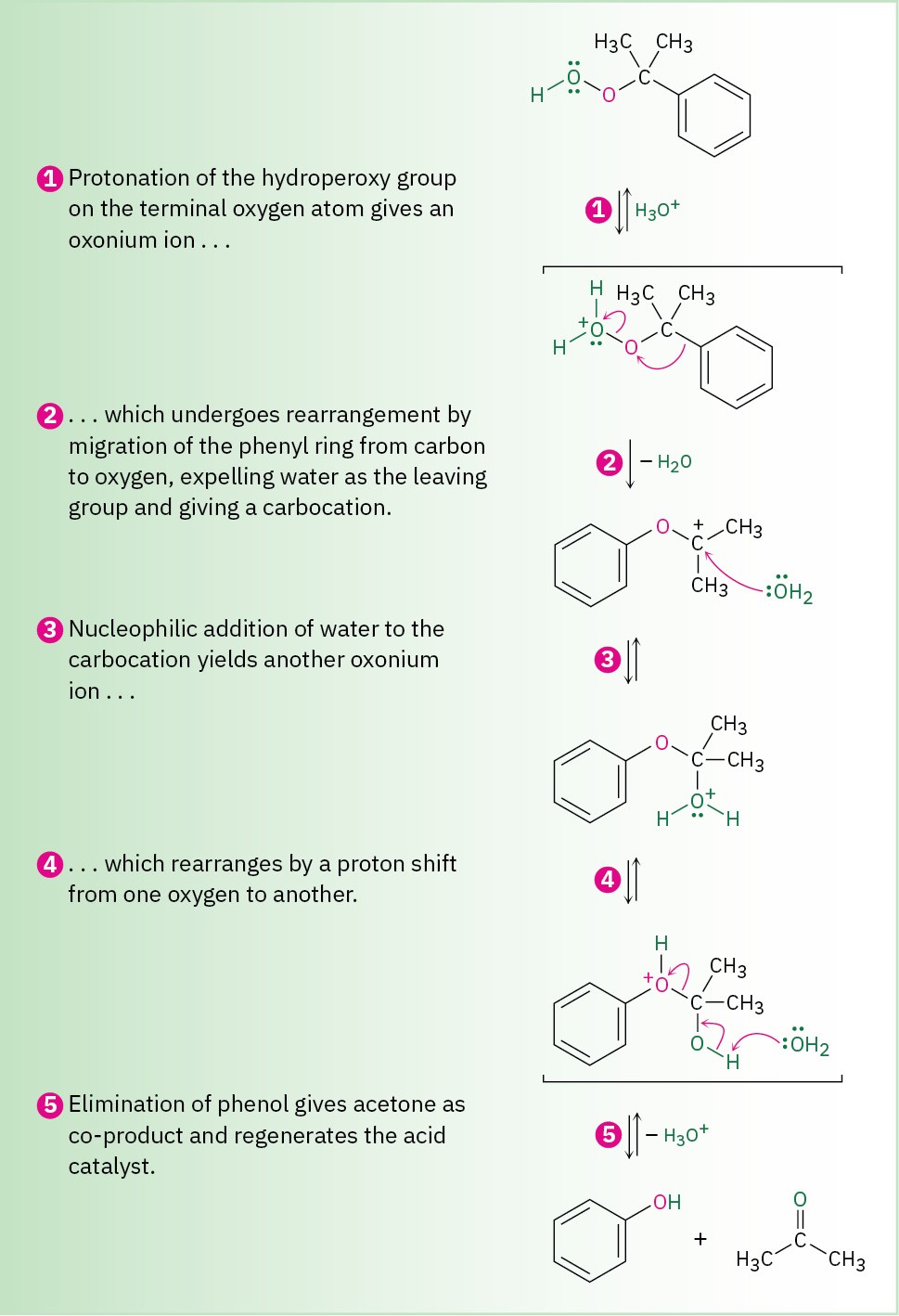
As shown in Figure 17.11, the reaction occurs by protonation of oxygen followed by shift of the phenyl group from carbon to oxygen with simultaneous loss of water. Replacement of the water then yields an intermediate called a hemiacetal—a compound that contains one – OR group and one –OH group bonded to the same carbon atom—which breaks down to phenol and acetone.
Figure 17.11 MECHANISM
Mechanism for the formation of phenol by acid-catalyzed rearrangement of cumene hydroperoxide.
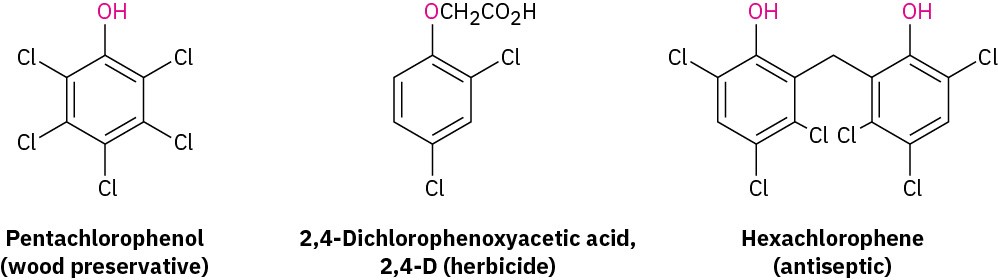
In addition to its use in making resins and adhesives, phenol is also the starting material for the synthesis of chlorinated phenols and the food preservatives BHT (butylated hydroxytoluene) and BHA (butylated hydroxyanisole). Pentachlorophenol, a widely used wood preservative, is prepared by reaction of phenol with excess Cl2. The herbicide 2,4-D (2,4-dichlorophenoxyacetic acid) is prepared from 2,4-dichlorophenol, and the hospital antiseptic agent hexachlorophene is prepared from 2,4,5-trichlorophenol.
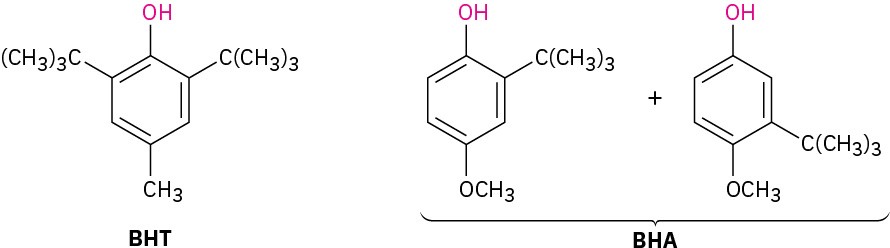
The food preservative BHT is prepared by Friedel–Crafts alkylation of p-methylphenol (p– cresol) with 2-methylpropene in the presence of acid; BHA is prepared similarly by alkylation of p-methoxyphenol.
Problem 17-17
Show the mechanism for the reaction of p-methylphenol with 2-methylpropene and H3PO4 catalyst to yield the food additive BHT.

