Additional Problems 5
Visualizing Chemistry
Problem 5-26
Which of the following structures are identical? (Green = Cl.)
(a) 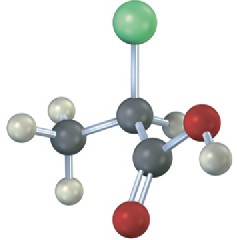 (b)
(b) 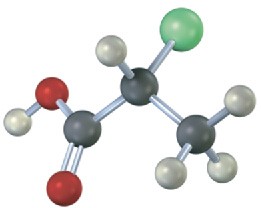 (c)
(c) 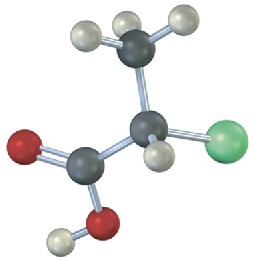 (d)
(d) 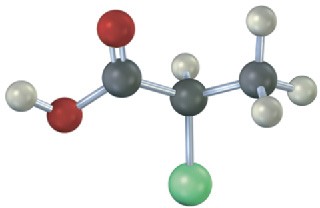
Problem 5-27
Assign R or S configurations to the chirality centers in the following molecules (blue = N):
(a)
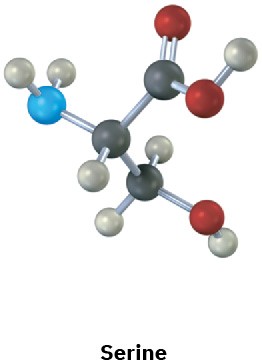
(b)
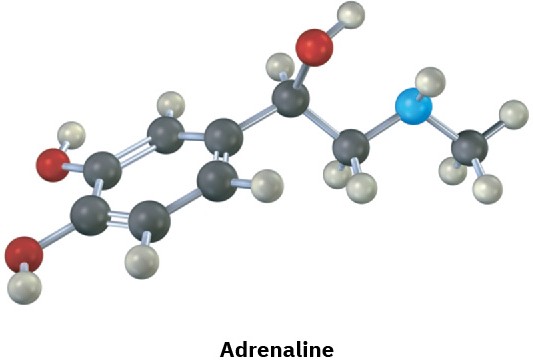
Problem 5-28
Which, if any, of the following structures represent meso compounds? (Blue = N, green = Cl.)
(a)
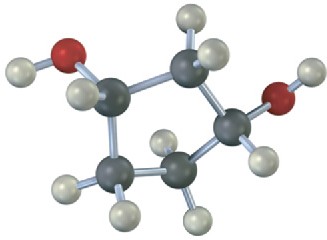
(b)
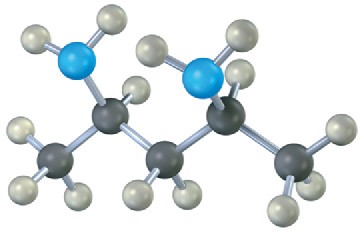
(c)
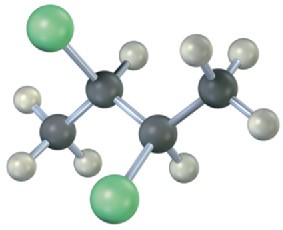
Problem 5-29
Assign R or S configuration to each chirality center in pseudoephedrine, an over-the- counter decongestant found in cold remedies (blue = N).
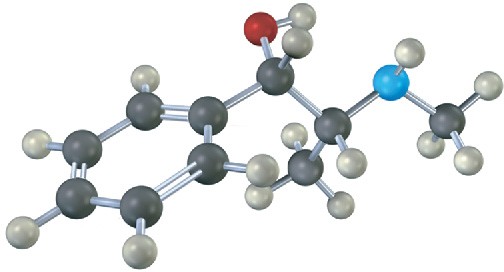
Problem 5-30
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
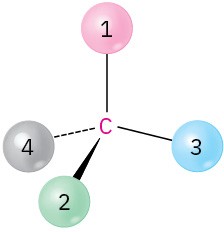
(b)
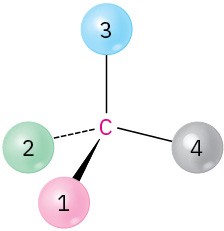
(c)
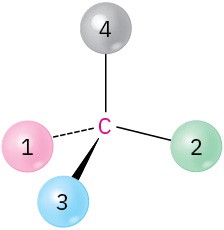
Chirality and Optical Activity
Problem 5-31
Which of the following objects are chiral?
(a) A basketball (b) A fork (c) A wine glass (d) A golf club( e) A spiral staircase (f) A snowflake
Problem 5-32
Which of the following compounds are chiral? Draw them, and label the chirality centers.
(a) 2,4-Dimethylheptane
(b) 5-Ethyl-3,3-dimethylheptane
(c) cis-1,4-Dichlorocyclohexane
Problem 5-33
Draw chiral molecules that meet the following descriptions:
(a) A chloroalkane, C5H11Cl
(b) An alcohol, C6H14O
(c) An alkene, C6H12
(d) An alkane, C8H18
Problem 5-34
Eight alcohols have the formula C5H12O. Draw them. Which are chiral?
Problem 5-35
Draw compounds that fit the following descriptions:
(a) A chiral alcohol with four carbons
(b) A chiral carboxylic acid with the formula C5H10O2
(c) A compound with two chirality centers
(d) A chiral aldehyde with the formula C3H5BrO
Problem 5-36
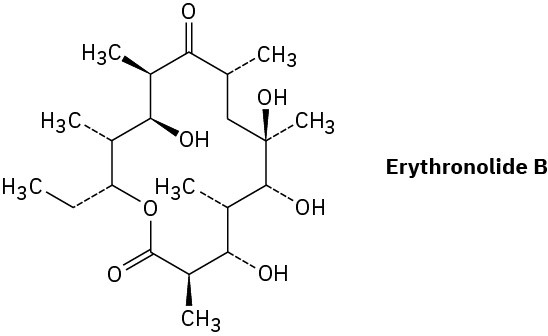
Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How many chirality centers does erythronolide B have? Identify them.
Assigning Configuration to Chirality Centers
Problem 5-37
Which of the following pairs of structures represent the same enantiomer, and which represent different enantiomers?
(a)
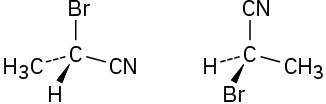
(b)
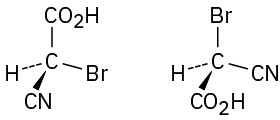
(c)
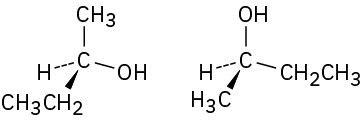
(d)
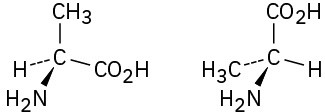
Problem 5-38
What is the relationship between the specific rotations of (2R,3R)-dichloropentane and (2S,3S)-dichloropentane? Between (2R,3S)-dichloropentane and (2R,3R)-dichloropentane?
Problem 5-39
What is the stereochemical configuration of the enantiomer of (2S,4R)-2,4-octanediol? Problem 5-40
What are the stereochemical configurations of the two diastereomers of (2S,4R)-2,4- octanediol? (A diol is a compound with two –OH groups.)
Problem 5-41
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
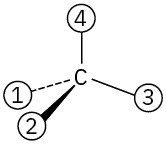
(b)
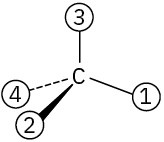
(c)
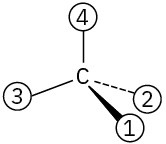
Problem 5-42
Assign Cahn–Ingold–Prelog rankings to the following sets of substituents:
(a)
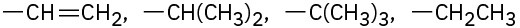
(b)
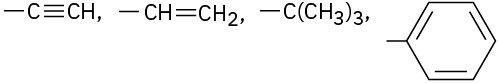
(c)
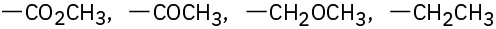
(d)
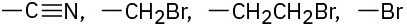
Problem 5-43
Assign R or S configurations to each chirality center in the following molecules:
(a)
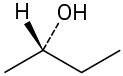
(b)
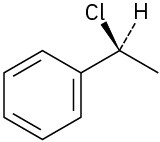
(c)
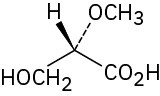
Problem 5-44
Assign R or S configuration to each chirality center in the following molecules:
(a)
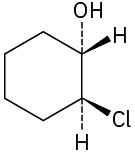
(b)
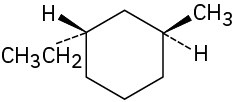
(c)
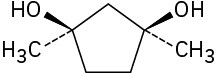
Problem 5-45
Assign R or S configuration to each chirality center in the following biological molecules:
(a)
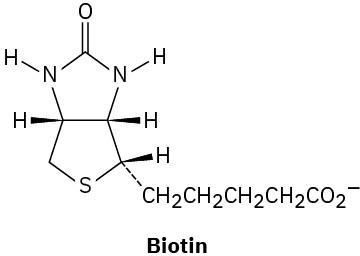
(b)
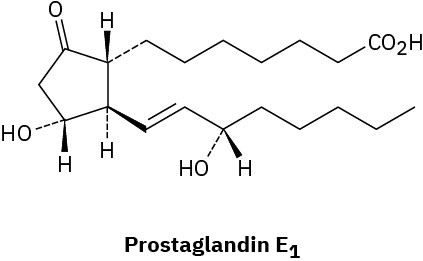
Problem 5-46
Draw tetrahedral representations of the following molecules:
(a) (S)-2-Chlorobutane
(b) (R)-3-Chloro-1-pentene [H2C=CHCH(Cl)CH2CH3]
Problem 5-47
Assign R or S configuration to each chirality center in the following molecules:
(a)
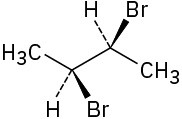
(b)
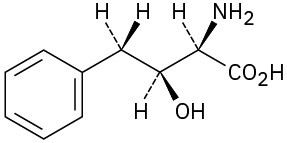
Problem 5-48
Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C).
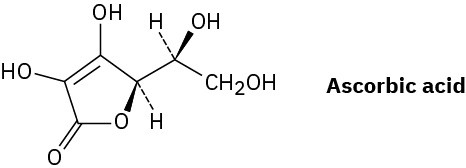
Problem 5-49
Assign R or S stereochemistry to the chirality centers in the following Newman projections:
(a)
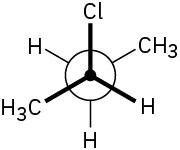
(b)
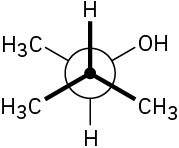
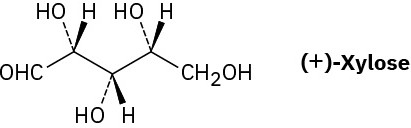
Problem 5-50
Xylose is a common sugar found in many types of wood, including maple and cherry. Because it is much less prone to cause tooth decay than sucrose, xylose has been used in candy and chewing gum. Assign R or S configurations to the chirality centers in xylose.
Meso Compounds
Problem 5-51
Draw examples of the following:
(a) A meso compound with the formula C8H18
(b) A meso compound with the formula C9H20
(c) A compound with two chirality centers, one R and the other S
Problem 5-52
Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each:
(a)
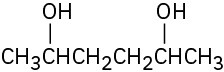
(b)
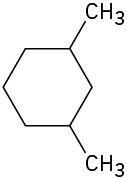
(c)
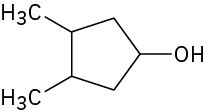
Problem 5-53
Draw the structure of a meso compound that has five carbons and three chirality centers.
Problem 5-54
Ribose, an essential part of ribonucleic acid (RNA), has the following structure:
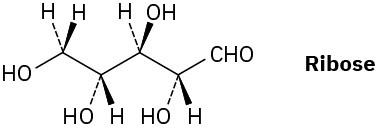
(a) How many chirality centers does ribose have? Identify them.
(b) How many stereoisomers of ribose are there?
(c) Draw the structure of the enantiomer of ribose.
(d) Draw the structure of a diastereomer of ribose.
Problem 5-55
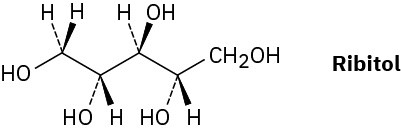
On reaction with hydrogen gas in the presence of a platinum catalyst, ribose (Problem 5- 54) is converted into ribitol. Is ribitol optically active or inactive? Explain.
Prochirality
Problem 5-56
Identify the indicated hydrogens in the following molecules as pro-R or pro-S:
(a)
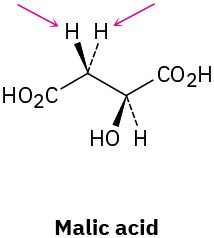
(b)
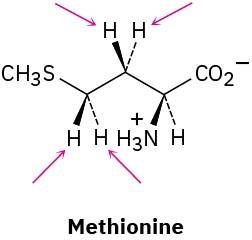
(c)
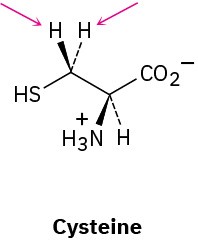
Problem 5-57
Identify the indicated faces in the following molecules as Re or Si:
(a)
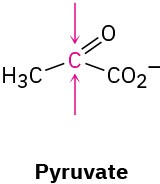
(b)
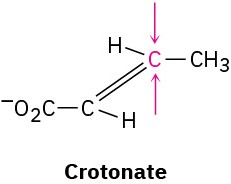
Problem 5-58
One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. The reaction occurs by addition of –OH to the Si face at C3, followed by protonation at C2, also from the Si face. Draw the product of the reaction, showing the stereochemistry of each step.
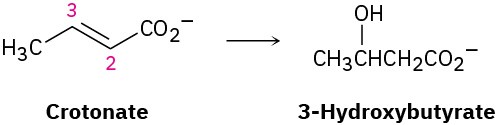
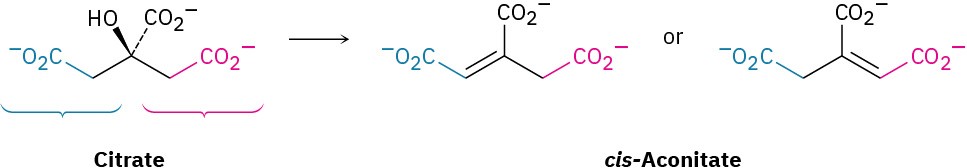
Problem 5-59
The dehydration of citrate to yield cis-aconitate, a step in the citric acid cycle, involves the pro-R “arm” of citrate rather than the pro-S arm. Which of the following two products is formed?
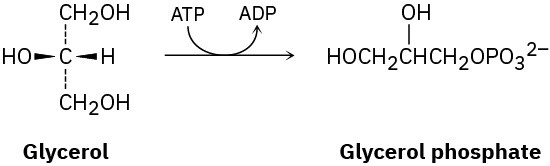
Problem 5-60
The first step in the metabolism of glycerol, formed by digestion of fats, is phosphorylation of the pro-R –CH2OH group by reaction with adenosine triphosphate (ATP) to give the corresponding glycerol phosphate plus adenosine diphosphate (ADP). Show the stereochemistry of the product.
Problem 5-61
One of the steps in fatty-acid biosynthesis is the dehydration of (R)-3-hydroxybutyryl ACP to give trans-crotonyl ACP. Does the reaction remove the pro-R or the pro-S hydrogen from C2?
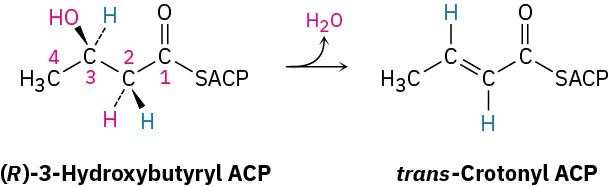
General Problems
Problem 5-62
Draw all possible stereoisomers of 1,2-cyclobutanedicarboxylic acid, and indicate the interrelationships. Which, if any, are optically active? Do the same for 1,3- cyclobutanedicarboxylic acid.
Problem 5-63
Draw tetrahedral representations of the two enantiomers of the amino acid cysteine, HSCH2CH(NH2)CO2H, and identify each as R or S.
Problem 5-64
The naturally occurring form of the amino acid cysteine (Problem 5-63) has the R configuration at its chirality center. On treatment with a mild oxidizing agent, two cysteines join to give cystine, a disulfide. Assuming that the chirality center is not affected by the reaction, is cystine optically active? Explain.
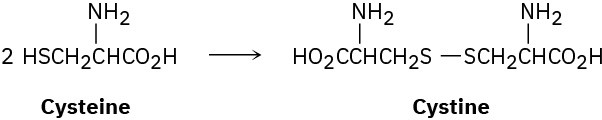
Problem 5-65
Draw tetrahedral representations of the following molecules:
(a) The 2S,3R enantiomer of 2,3-dibromopentane
(b) The meso form of 3,5-heptanediol
Problem 5-66
Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States.
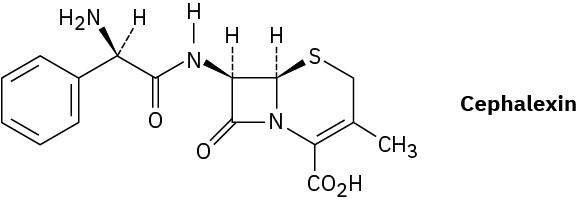
Problem 5-67
Chloramphenicol, a powerful antibiotic isolated in 1947 from the Streptomyces venezuelae bacterium, is active against a broad spectrum of bacterial infections and is particularly valuable against typhoid fever. Assign R or S configurations to the chirality centers in chloramphenicol.
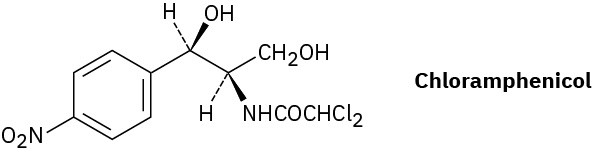
Problem 5-68
Allenes are compounds with adjacent carbon–carbon double bonds. Many allenes are chiral, even though they don’t contain chirality centers. Mycomycin, for example, a naturally occurring antibiotic isolated from the bacterium Nocardia acidophilus, is chiral and has [α]D
= −130. Explain why mycomycin is chiral.
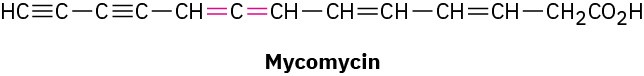
Problem 5-69
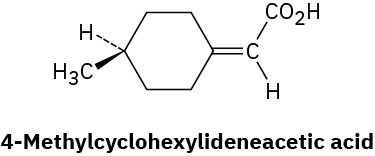
Long before chiral allenes were known (Problem 5-68), the resolution of 4- methylcyclohexylideneacetic acid into two enantiomers had been carried out. Why is it chiral? What geometric similarity does it have to allenes?
Problem 5-70
(S)-1-Chloro-2-methylbutane undergoes light-induced reaction with Cl2 to yield a mixture of products, among which are 1,4-dichloro-2-methylbutane and 1,2-dichloro-2- methylbutane.
(a) Write the reaction, showing the correct stereochemistry of the reactant.
(b) One of the two products is optically active, but the other is optically inactive. Which is which?
Problem 5-71
How many stereoisomers of 2,4-dibromo-3-chloropentane are there? Draw them, and indicate which are optically active.
Problem 5-72
Draw both cis– and trans-1,4-dimethylcyclohexane in their more stable chair conformations.
(a) How many stereoisomers are there of cis-1,4-dimethylcyclohexane, and how many of trans– 1,4-dimethylcyclohexane?
(b) Are any of the structures chiral?
(c) What are the stereochemical relationships among the various stereoisomers of 1,4- dimethylcyclohexane?
Problem 5-73
Draw both cis– and trans-1,3-dimethylcyclohexane in their more stable chair conformations.
(a) How many stereoisomers are there of cis-1,3-dimethylcyclohexane, and how many of trans– 1,3-dimethylcyclohexane?
(b) Are any of the structures chiral?
(c) What are the stereochemical relationships among the various stereoisomers of 1,3- dimethylcyclohexane?
Problem 5-74
cis-1,2-Dimethylcyclohexane is optically inactive even though it has two chirality centers. Explain.
Problem 5-75
We’ll see in Chapter 11 that alkyl halides react with hydrosulfide ion (HS−) to give a product whose stereochemistry is inverted from that of the reactant.
Draw the reaction of (S)-2-bromobutane with HS− ion to yield 2-butanethiol, CH3CH2CH(SH)CH3. Is the stereochemistry of the product R or S?
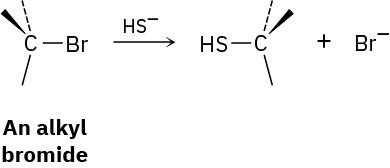
Problem 5-76
Ketones react with sodium acetylide (the sodium salt of acetylene, Na+– : C≡CH) to give alcohols. For example, the reaction of sodium acetylide with 2-butanone yields 3-methyl-1- pentyn-3-ol:
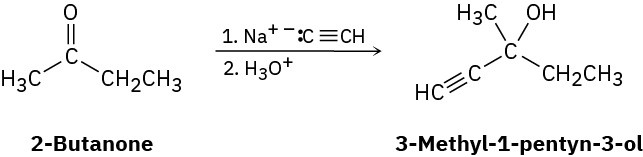
(a) Is the product chiral?
(b) Assuming that the reaction takes place with equal likelihood from both Re and Si faces of the carbonyl group, is the product optically active? Explain.
Problem 5-77
Imagine that a reaction similar to that in Problem 5-76 is carried out between sodium acetylide and (R)-2-phenylpropanal to yield 4-phenyl-1-pentyn-3-ol:
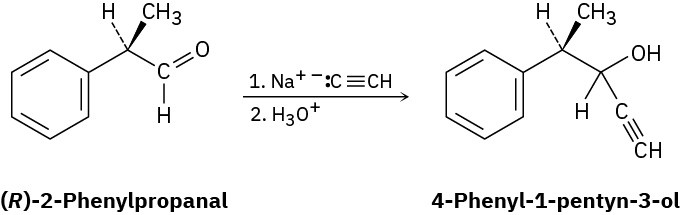
(a) Is the product chiral?
(b) Draw both major and minor reaction products, assuming that the reaction takes place preferentially from the Re face of the carbonyl group. Is the product mixture optically active? Explain.

