9.9 An Introduction to Organic Synthesis
As mentioned in the introduction, one of the purposes of this chapter is to use alkyne chemistry as a vehicle to begin looking at some of the general strategies used in organic synthesis—the construction of complex molecules in the laboratory. There are many reasons for carrying out the laboratory synthesis of an organic compound. In the pharmaceutical industry, new molecules are designed and synthesized in the hope that some might be useful new drugs. In the chemical industry, syntheses are done to devise more economical routes to known compounds. In academic laboratories, the synthesis of extremely complex molecules is sometimes done just for the intellectual challenge involved in mastering so difficult a subject. The successful synthesis route is a highly creative work that is sometimes described by such subjective terms as elegant or beautiful.
In this book, too, we will often devise syntheses of molecules from simpler precursors, but the purpose here is to learn. The ability to plan a successful multistep synthetic sequence requires a working knowledge of the uses and limitations of many different organic reactions. Furthermore, it requires the practical ability to piece together the steps in a sequence such that each reaction does only what is desired without causing changes elsewhere in the molecule. Planning a synthesis makes you approach a chemical problem in a logical way, draw on your knowledge of chemical reactions, and organize that knowledge into a workable plan—it helps you learn organic chemistry.
There’s no secret to planning an organic synthesis: all it takes is a knowledge of the different reactions and some practice. The only real trick is to work backward in what is often called a retrosynthetic direction. Don’t look at a potential starting material and ask yourself what reactions it might undergo. Instead, look at the final product and ask, “What was the immediate precursor of that product?” For example, if the final product is an alkyl halide, the immediate precursor might be an alkene, to which you could add HX. If the final product is a cis alkene, the immediate precursor might be an alkyne, which you could hydrogenate using the Lindlar catalyst. Having found an immediate precursor, work backward again, one step at a time, until you get back to the starting material. You have to keep the starting material in mind, of course, so that you can work back to it, but you don’t want that starting material to be your main focus.
Let’s work several examples of increasing complexity.
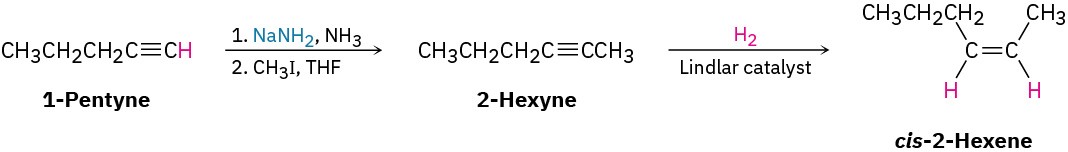
Worked Example 9.1Devising a Synthesis RouteHow would you synthesize cis-2-hexene from 1-pentyne and an alkyl halide? More than one step is needed.
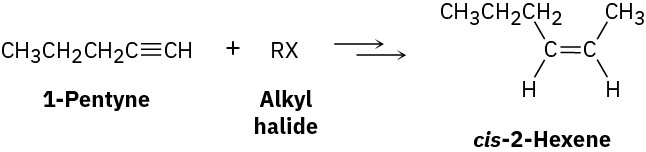
StrategyWhen undertaking any synthesis problem, you should look at the product, identify the functional groups it contains, and then ask yourself how those functional groups can be prepared. Always work retrosynthetically, one step at a time.The product in this case is a cis-disubstituted alkene, so the first question is, “What is an immediate precursor of a cis-disubstituted alkene?” We know that an alkene can be prepared from an alkyne by reduction and that the right choice of experimental conditions will allow us to prepare either a trans-disubstituted alkene (using lithium in liquid ammonia) or a cis-disubstituted alkene (using catalytic hydrogenation over the Lindlar catalyst). Thus, reduction of 2-hexyne by catalytic hydrogenation using the Lindlar catalyst should yield cis-2-hexene.
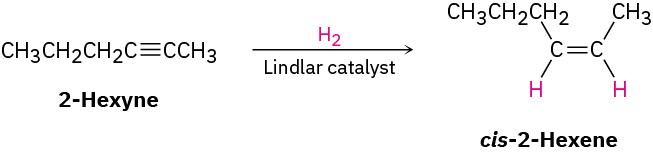
Next ask, “What is an immediate precursor of 2-hexyne?” We’ve seen that an internal alkyne can be prepared by alkylation of a terminal alkyne anion. In the present instance, we’re told to start with 1-pentyne and an alkyl halide. Thus, alkylation of the anion of 1- pentyne with iodomethane should yield 2-hexyne.
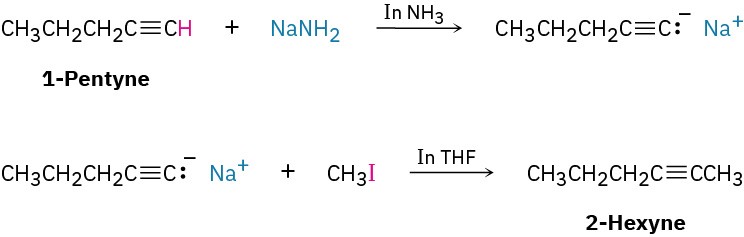 Solutioncis-2-Hexene can be synthesized from the given starting materials in three steps.
Solutioncis-2-Hexene can be synthesized from the given starting materials in three steps.
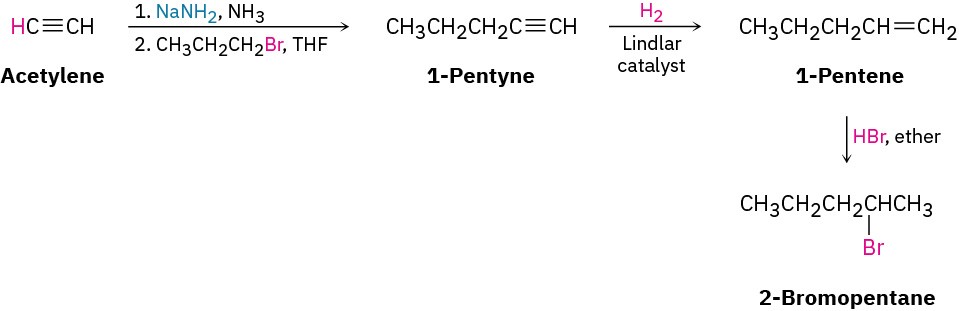
Worked Example 9.2Devising a Synthesis RouteHow would you synthesize 2-bromopentane from acetylene and an alkyl halide? More than one step is needed.
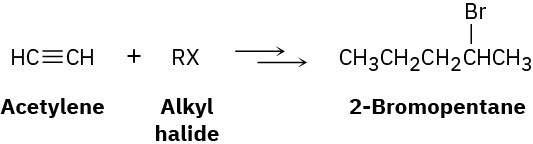 StrategyIdentify the functional group in the product (an alkyl bromide) and work the problem retrosynthetically. What is an immediate precursor of an alkyl bromide? Perhaps an alkene plus HBr. Of the two possibilities, Markovnikov addition of HBr to 1-pentene looks like a better choice than addition to 2-pentene because the latter reaction would give a mixture of isomers.
StrategyIdentify the functional group in the product (an alkyl bromide) and work the problem retrosynthetically. What is an immediate precursor of an alkyl bromide? Perhaps an alkene plus HBr. Of the two possibilities, Markovnikov addition of HBr to 1-pentene looks like a better choice than addition to 2-pentene because the latter reaction would give a mixture of isomers.
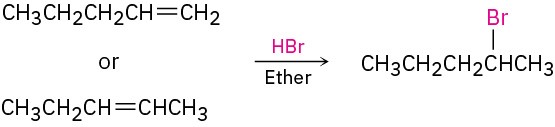
What is an immediate precursor of an alkene? Perhaps an alkyne, which could be reduced.

What is an immediate precursor of a terminal alkyne? Perhaps sodium acetylide and an alkyl halide.
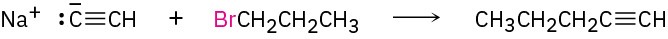 SolutionThe desired product can be synthesized in four steps from acetylene and 1-bromopropane.
SolutionThe desired product can be synthesized in four steps from acetylene and 1-bromopropane.
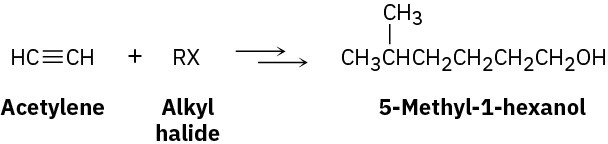
Worked Example 9.3Devising a Synthesis RouteHow would you synthesize 5-methyl-1-hexanol (5-methyl-1-hydroxyhexane) from acetylene and an alkyl halide?
 StrategyWhat is an immediate precursor of a primary alcohol? Perhaps a terminal alkene, which could be hydrated with non-Markovnikov regiochemistry by reaction with borane followed by oxidation with H2O2.
StrategyWhat is an immediate precursor of a primary alcohol? Perhaps a terminal alkene, which could be hydrated with non-Markovnikov regiochemistry by reaction with borane followed by oxidation with H2O2.
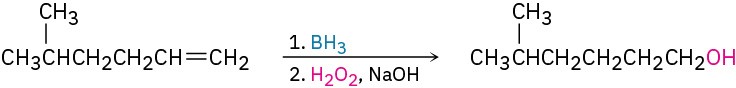
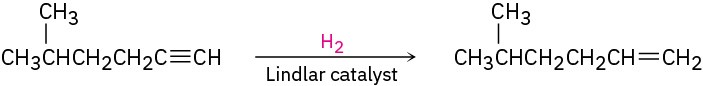
What is an immediate precursor of a terminal alkene? Perhaps a terminal alkyne, which could be reduced.
What is an immediate precursor of 5-methyl-1-hexyne? Perhaps acetylene and 1-bromo-3- methylbutane.
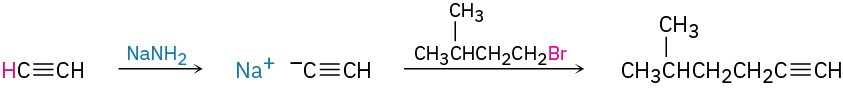 SolutionThe synthesis can be completed in four steps from acetylene and 1-bromo-3-methylbutane:
SolutionThe synthesis can be completed in four steps from acetylene and 1-bromo-3-methylbutane:
Problem 9-12
Beginning with 4-octyne as your only source of carbon, and using any inorganic reagents necessary, how would you synthesize the following compounds?
(a)
cis-4-Octene (b)
Butanal (c)
- Bromooctane (d)
4-Octanol (e)
4,5-Dichlorooctane (f)
Butanoic acid Problem 9-13
Beginning with acetylene and any alkyl halide needed, how would you synthesize the following compounds?
(a) Decane (b)
2,2-Dimethylhexane (c)
Hexanal (d)
2-Heptanone
Additional Problems 9 • Additional Problems 9 • Additional Problems Visualizing Chemistry Problem 9-14
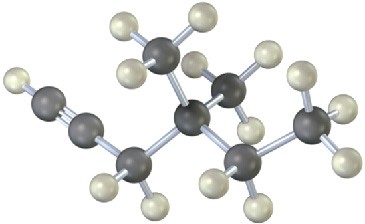
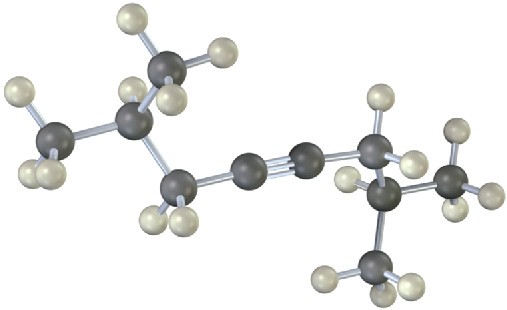
Name the following alkynes, and predict the products of their reaction with (1) H2 in the presence of a Lindlar catalyst and (2) H3O+ in the presence of HgSO4:
(a)
(b)
Problem 9-15
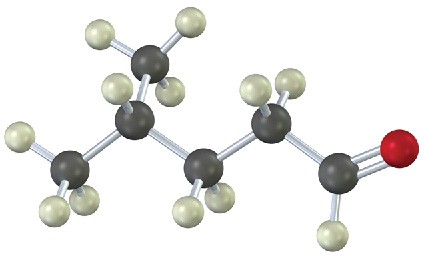 From what alkyne might each of the following substances have been made? (Green = Cl.) (a)
From what alkyne might each of the following substances have been made? (Green = Cl.) (a)
(b)
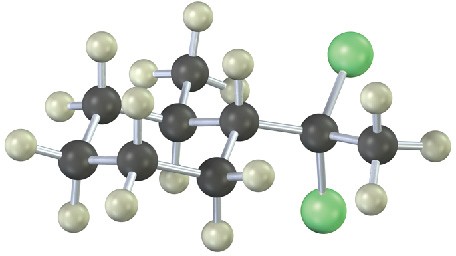
Problem 9-16
How would you prepare the following substances, starting from any compounds having four carbons or fewer?
(a)
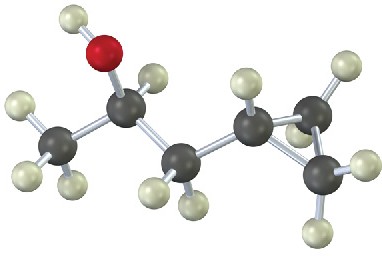
(b)
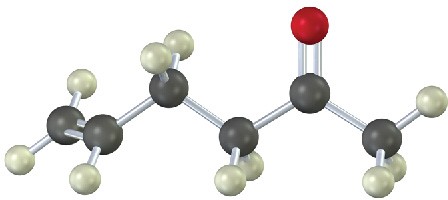
Problem 9-17
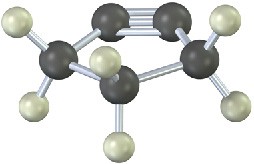
The following cycloalkyne is too unstable to exist. Explain.
Mechanism Problems
Problem 9-18
Assuming that halogens add to alkynes in the same manner as they add to alkenes, propose a mechanism for and predict the product(s) of the reaction of phenylpropyne with Br2.
Problem 9-19
Assuming that strong acids add to alkynes in the same manner as they add to alkenes, propose a mechanism for each of the following reactions.
(a)
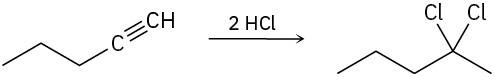
(b)
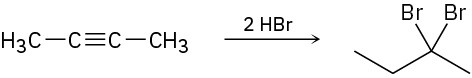
(c)
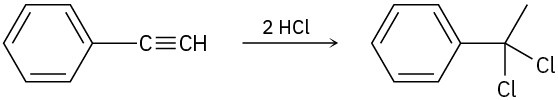
Problem 9-20
The mercury-catalyzed hydration of alkynes involves the formation of an organomercury enol intermediate. Draw the electron-pushing mechanism to show how each of the following intermediates is formed.
(a)
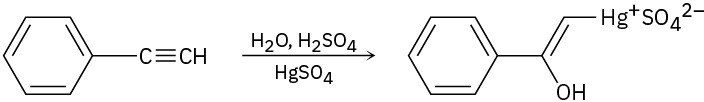
(b)
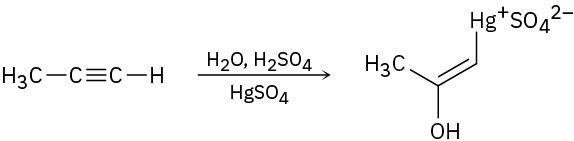
(c)
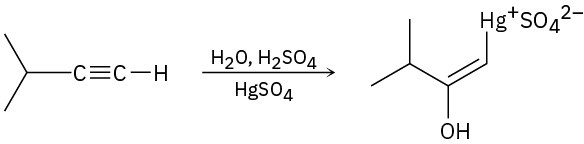
Problem 9-21
The final step in the hydration of an alkyne under acidic conditions is the tautomerization of an enol intermediate to give the corresponding ketone. The mechanism involves a protonation followed by a deprotonation. Show the mechanism for each of the following tautomerizations.
(a)
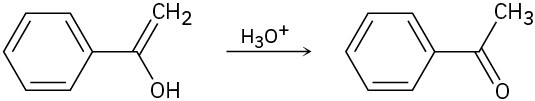
(b)
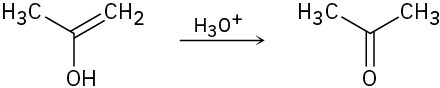
(c)
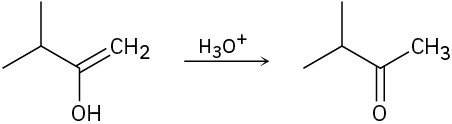
Problem 9-22
Predict the product(s) and show the complete electron-pushing mechanism for each of the following dissolving metal reductions.
(a)
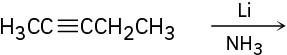
(b)
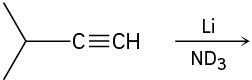
(c)
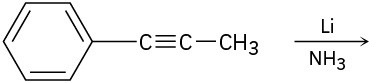
Problem 9-23
Identify the mechanisms for the following reactions as polar, radical, or both. (a)
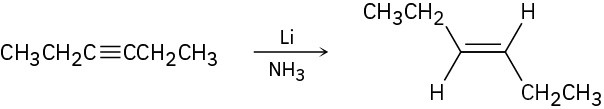
(b)
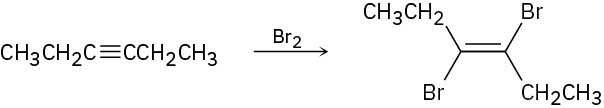
(c)
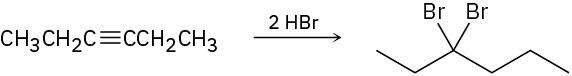
Problem 9-24
Predict the product and provide the complete electron-pushing mechanism for the following two-step synthetic processes.
(a)
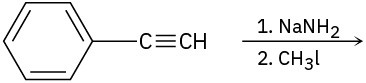
(b)
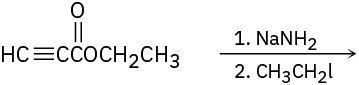
(c)
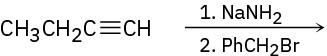
Problem 9-25
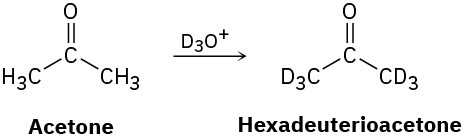
Reaction of acetone with D3O+ yields hexadeuterioacetone. That is, all the hydrogens in acetone are exchanged for deuterium. Review the mechanism of mercuric-ion-catalyzed alkyne hydration, and then propose a mechanism for this deuterium incorporation.
Naming Alkynes
Problem 9-26
Give IUPAC names for the following compounds: (a)
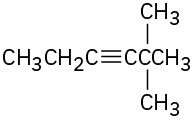
(b)
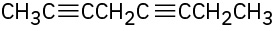
(c)
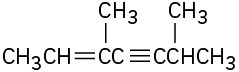
(d)
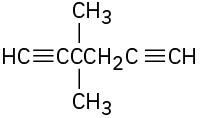
(e)
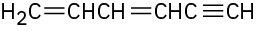
(f)
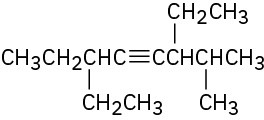
Problem 9-27
Draw structures corresponding to the following names:
(a)
3,3-Dimethyl-4-octyne (b)
3-Ethyl-5-methyl-1,6,8-decatriyne (c)
2,2,5,5-Tetramethyl-3-hexyne (d)
3,4-Dimethylcyclodecyne (e)
3,5-Heptadien-1-yne (f)
3-Chloro-4,4-dimethyl-1-nonen-6-yne (g)
3-sec–Butyl-1-heptyne (h)
5-tert-Butyl-2-methyl-3-octyne Problem 9-28
The following two hydrocarbons have been isolated from various plants in the sunflower family. Name them according to IUPAC rules.
(a)
CH3CH=CHC≡CC≡CCH=CHCH=CHCH=CH2 (all trans)
(b) CH3C≡CC≡CC≡CC≡CC≡CCH=CH2
Reactions of Alkynes
Problem 9-29
Terminal alkynes react with Br2 and water to yield bromo ketones. For example:
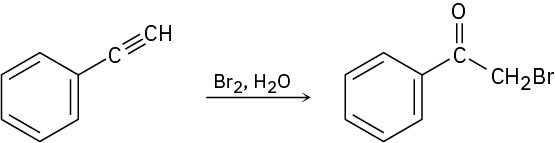
Propose a mechanism for the reaction. To what reaction of alkenes is the process analogous?
Problem 9-30
Predict the products of the following reactions:
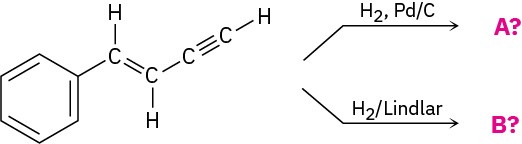
Problem 9-31
Predict the products from reaction of 1-hexyne with the following reagents: (a)
1 equiv HBr (b)
1 equiv Cl2 (c)
H2, Lindlar catalyst (d)
NaNH2 in NH3, then CH3Br (e)
H2O, H2SO4, HgSO4
(f)
2 equiv HCl
Problem 9-32
Predict the products from reaction of 5-decyne with the following reagents: (a)
H2, Lindlar catalyst (b)
Li in NH3 (c)
1 equiv Br2 (d)
BH3 in THF, then H2O2, OH−
(e)
H2O, H2SO4, HgSO4
(f)
Excess H2, Pd/C catalyst Problem 9-33
Predict the products from reaction of 2-hexyne with the following reagents: (a)
2 equiv Br2 (b)
1 equiv HBr (c)
Excess HBr (d)
Li in NH3 (e)
H2O, H2SO4, HgSO4
Problem 9-34
Propose structures for hydrocarbons that give the following products on oxidative cleavage by KMnO4 or O3:
(a)
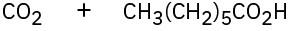
(b)
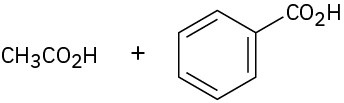
(c)
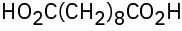
(d)
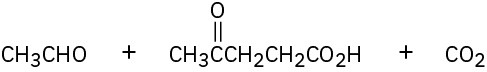
(e)
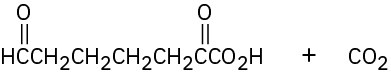
Problem 9-35
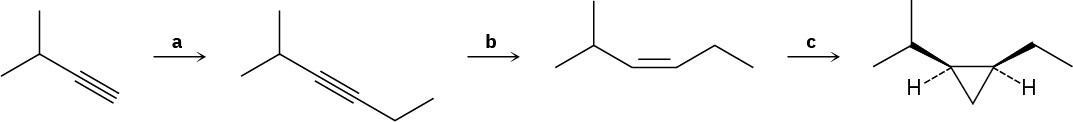
Identify the reagents a–c in the following scheme:
Organic Synthesis
Problem 9-36
How would you carry out the following multistep conversions? More than one step may be needed in some instances.
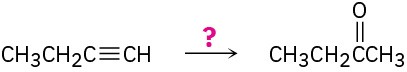
Problem 9-37
How would you carry out the following reactions? (a)
(b)
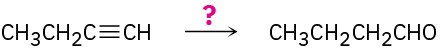
(c)
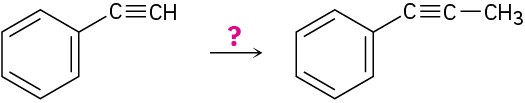
(d)
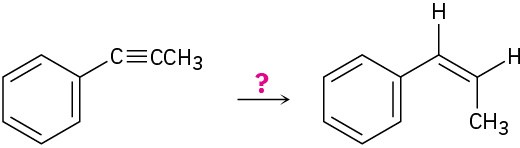
(e)
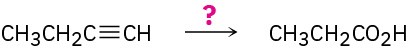
(f)

Problem 9-38
Each of the following syntheses requires more than one step. How would you carry them out?
(a)
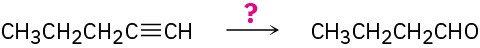
(b)
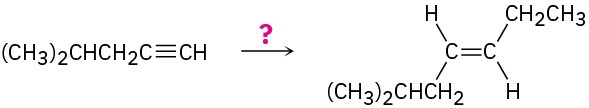
Problem 9-39
How would you carry out the following multistep transformation?
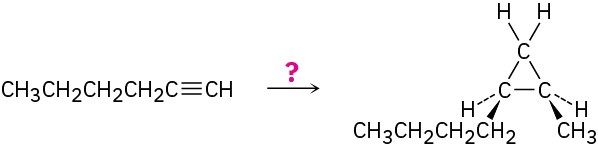
Problem 9-40
How would you carry out the following multistep conversions?
Problem 9-41
Synthesize the following compounds using 1-butyne as the only source of carbon, along with any inorganic reagents you need. More than one step may be needed.
(a)
1,1,2,2-Tetrachlorobutane (b)
1,1-Dichloro-2-ethylcyclopropane Problem 9-42
How would you synthesize the following compounds from acetylene and any alkyl halides with four or fewer carbons? More than one step may be needed.
(a)

(b)
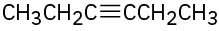
(c)
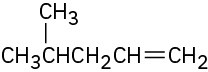
(d)
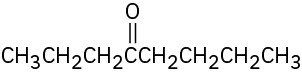
(e)
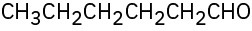
Problem 9-43
How would you carry out the following reactions to introduce deuterium into organic molecules?
(a)
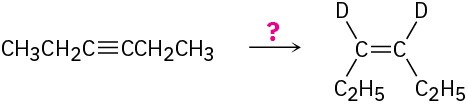
(b)
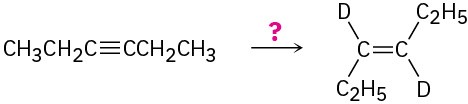
(c)

(d)
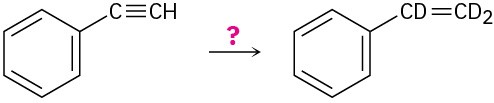
Problem 9-44
How would you prepare cyclodecyne starting from acetylene and any required alkyl halide?
Problem 9-45
The sex attractant given off by the common housefly is an alkene named muscalure. Propose a synthesis of muscalure starting from acetylene and any alkyl halides needed. What is the IUPAC name for muscalure?
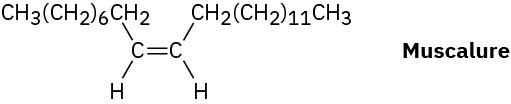
General Problems
Problem 9-46
A hydrocarbon of unknown structure has the formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a palladium catalyst, 3 equivalents of H2 are absorbed.
(a)
How many degrees of unsaturation are present in the unknown structure? (b)
How many triple bonds are present? (c)
How many double bonds are present? (d)
How many rings are present? (e)
Draw a structure that fits the data. Problem 9-47
Compound A (C9H12) absorbed 3 equivalents of H2 on catalytic reduction over a palladium catalyst to give B (C9H18). On ozonolysis, compound A gave, among other things, a ketone that was identified as cyclohexanone. On treatment with NaNH2 in NH3, followed by addition of iodomethane, compound A gave a new hydrocarbon, C (C10H14). What are the structures of A, B, and C?
Problem 9-48
Hydrocarbon A has the formula C12H8. It absorbs 8 equivalents of H2 on catalytic reduction over a palladium catalyst. On ozonolysis, only two products are formed: oxalic acid (HO2CCO2H) and succinic acid (HO2CCH2CH2CO2H). Write the reactions, and propose a structure for A.
Problem 9-49
Occasionally, a chemist might need to invert the stereochemistry of an alkene—that is, to convert a cis alkene to a trans alkene, or vice versa. There is no one-step method for doing an alkene inversion, but the transformation can be carried out by combining several reactions in the proper sequence. How would you carry out the following reactions?
(a)

(b)

Problem 9-50
Organometallic reagents such as sodium acetylide undergo an addition reaction with ketones, giving alcohols:
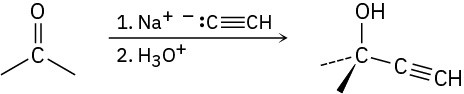
How might you use this reaction to prepare 2-methyl-1,3-butadiene, the starting material used in the manufacture of synthetic rubber?
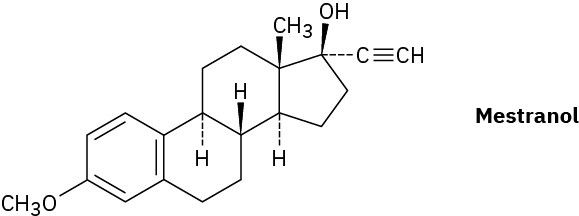
Problem 9-51
The oral contraceptive agent Mestranol is synthesized using a carbonyl addition reaction like that shown in Problem 9-50. Draw the structure of the ketone needed.
Problem 9-52
1-Octen-3-ol, a potent mosquito attractant commonly used in mosquito traps, can be prepared in two steps from hexanal, CH3CH2CH2CH2CH2CHO. The first step is an acetylide- addition reaction like that described in Problem 9-50. What is the structure of the product from the first step, and how can it be converted into 1-octen-3-ol?
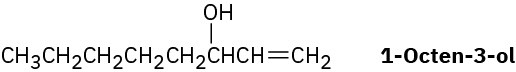
Problem 9-53
Erythrogenic acid, C18H26O2, is an acetylenic fatty acid that turns a vivid red on exposure to light. On catalytic hydrogenation over a palladium catalyst, 5 equivalents of H2 are absorbed, and stearic acid, CH3(CH2)16CO2H, is produced. Ozonolysis of erythrogenic acid gives four products: formaldehyde, CH2O; oxalic acid, HO2CCO2H; azelaic acid, HO2C(CH2)7CO2H; and the aldehyde acid OHC(CH2)4CO2H. Draw two possible structures for erythrogenic acid, and suggest a way to tell them apart by carrying out some simple reactions.
Problem 9-54
Hydrocarbon A has the formula C9H12 and absorbs 3 equivalents of H2 to yield B, C9H18, when hydrogenated over a Pd/C catalyst. On treatment of A with aqueous H2SO4 in the
presence of mercury(II), two isomeric ketones, C and D, are produced. Oxidation of A with KMnO4 gives a mixture of acetic acid (CH3CO2H) and the tricarboxylic acid E. Propose structures for compounds A–D, and write the reactions.
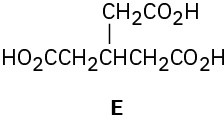
Problem 9-55
A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central carbon atoms have? What is the geometric relationship of the substituents on one end to the substituents on the other end? What kind of isomerism is possible? Make a model to help see the answer.
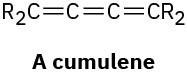
Problem 9-56
Which of the following bases could be used to deprotonate 1-butyne?

 (a)(b)
(a)(b) 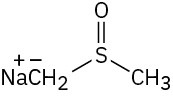 (c)(d)
(c)(d) 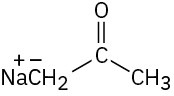
Problem 9-57
Arrange the following carbocations in order of increasing stability. (a)
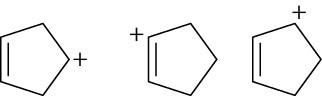
(b)
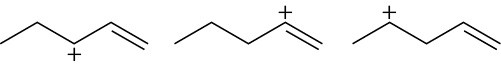
(c)