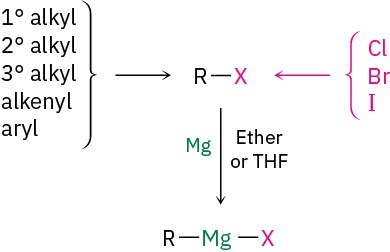
10.6 Reactions of Alkyl Halides: Grignard Reagents
Alkyl halides, RX, react with magnesium metal in ether or tetrahydrofuran (THF) solvent to yield alkylmagnesium halides, RMgX. The products, called Grignard reagents (RMgX) after their discoverer, Francois Auguste Victor Grignard, who received the 1912 Nobel Prize in Chemistry, are examples of organometallic compounds because they contain a carbon– metal bond. In addition to alkyl halides, Grignard reagents can also be made from alkenyl (vinylic) and aryl (aromatic) halides. The halogen can be Cl, Br, or I, although chlorides are less reactive than bromides and iodides. Organofluorides rarely react with magnesium.
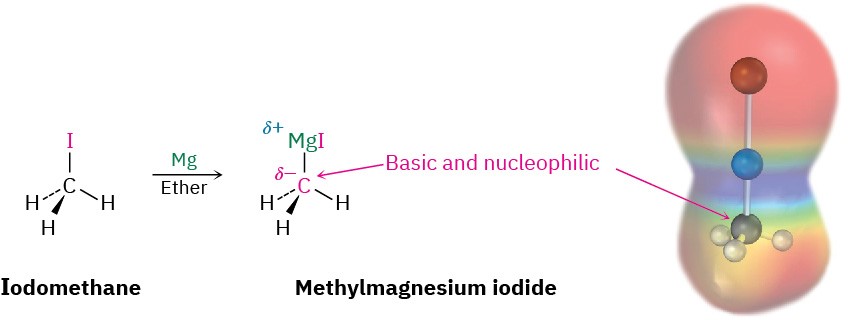
As you might expect from the discussion of electronegativity and bond polarity in Section 6.3, the carbon–magnesium bond is polarized, making the carbon atom of Grignard reagents both nucleophilic and basic. An electrostatic potential map of methylmagnesium iodide, for instance, indicates the electron-rich (red) character of the carbon bonded to magnesium.
A Grignard reagent is formally the magnesium salt, R3C–+MgX, of a carbon acid, R3C–H, and is thus a carbon anion, or carbanion. But because hydrocarbons are such weak acids, with pKa’s in the range 44 to 60 (Section 9.7), carbon anions are very strong bases. Grignard reagents must therefore be protected from atmospheric moisture to prevent their being protonated and destroyed in acid–base reactions: R–Mg–X + H2O → R–H + HO–Mg–X.
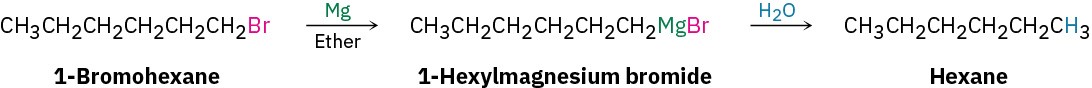
Grignard reagents themselves don’t occur in living organisms, but they serve as useful carbon-based nucleophiles in several important laboratory reactions, which we’ll look at in
detail in Section 17.5. In addition, they act as a simple model for other, more complex carbon-based nucleophiles that are important in biological chemistry. We’ll see many examples of these in Chapter 29.
Problem 10-9
How strong a base would you expect a Grignard reagent to be? Look at Table 9.1 and predict whether the following reactions will occur as written. (The pKa of NH3 is 35.)
(a) CH3MgBr + H–C≡C–H ⟶ CH4 + H–C≡C–MgBr(b) CH3MgBr + NH3 ⟶ CH4 + H2N– MgBr
Problem 10-10
How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a deuterated compound?


