20.5 Preparing Carboxylic Acids
Let’s review briefly some of the methods for preparing carboxylic acids that we’ve seen in previous chapters.
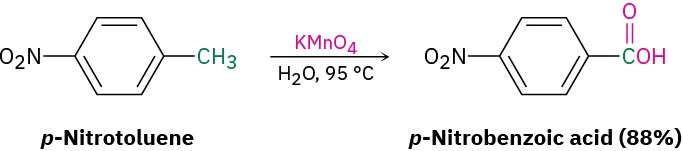
- Oxidation of a substituted alkylbenzene with KMnO4 gives a substituted benzoic acid (Section 16.8). Both primary and secondary alkyl groups can be oxidized, but tertiary groups are not affected.
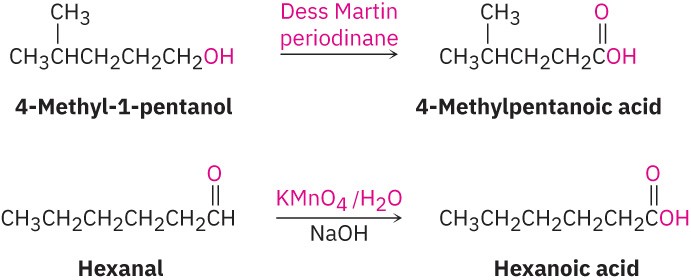
- Oxidation of a primary alcohol or an aldehyde yields a carboxylic acid (Section 17.7 and Section 19.3). Primary alcohols are often oxidized with the Dess Martin periodinane, and aldehydes are similarly oxidized with alkaline KMnO4.
Hydrolysis of Nitriles
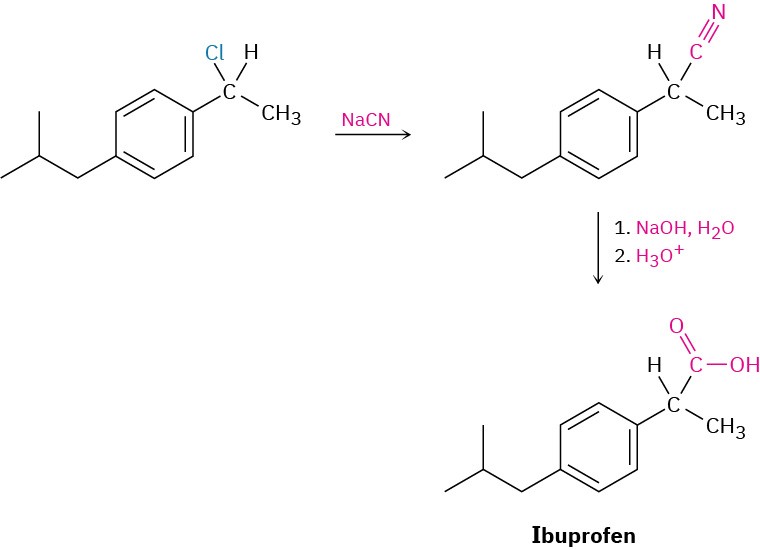
Carboxylic acids can be prepared from nitriles on heating with aqueous acid or base by a mechanism that we’ll discuss in Section 20.7. Since nitriles themselves are usually made by SN2 reaction of a primary or secondary alkyl halide with CN–, the two-step sequence of cyanide displacement followed by nitrile hydrolysis is a good way to make a carboxylic acid from an alkyl halide (RBr → RC≡N → RCO!H). Note that the product acid has one more carbon than the starting alkyl halide. One example occurs in a commercial route for the synthesis of the nonsteroidal anti-inflammatory drug ibuprofen. (See Chapter 15 Chemistry Matters.)
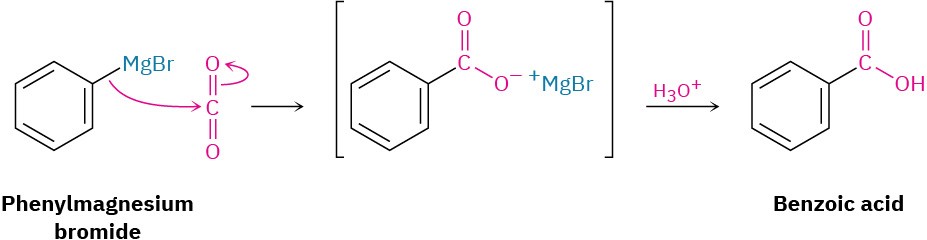
Carboxylation of Grignard Reagents
Another method for preparing carboxylic acids is by reaction of a Grignard reagent with CO2 to yield a metal carboxylate, followed by protonation to give a carboxylic acid. This carboxylation reaction is usually carried out by bubbling a stream of dry CO2 gas through a solution of the Grignard reagent. The organomagnesium halide adds to a C═O bond of carbon dioxide in a typical nucleophilic carbonyl addition reaction, and protonation of the carboxylate by addition of aqueous HCl in a separate step then gives the free carboxylic acid. For example:
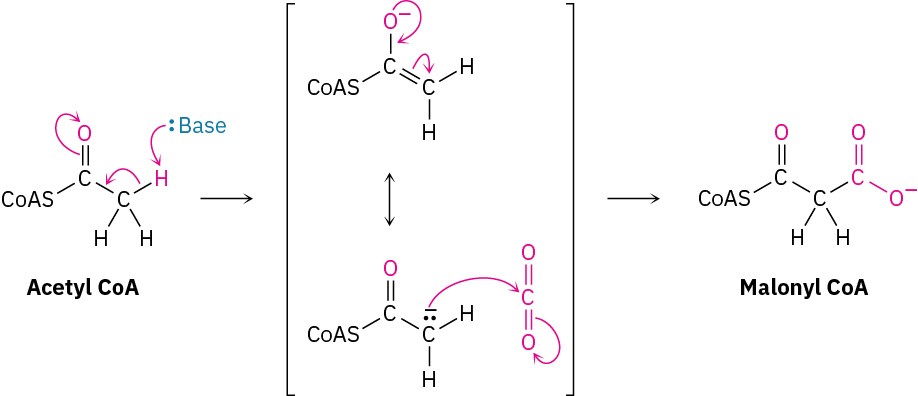
As noted previously, there are no Grignard reagents inside living cells, but there are other types of stabilized carbanions that are often carboxylated. One of the initial steps in fatty- acid biosynthesis, for instance, involves the formation of a carbanion from acetyl CoA, followed by carboxylation to yield malonyl CoA.
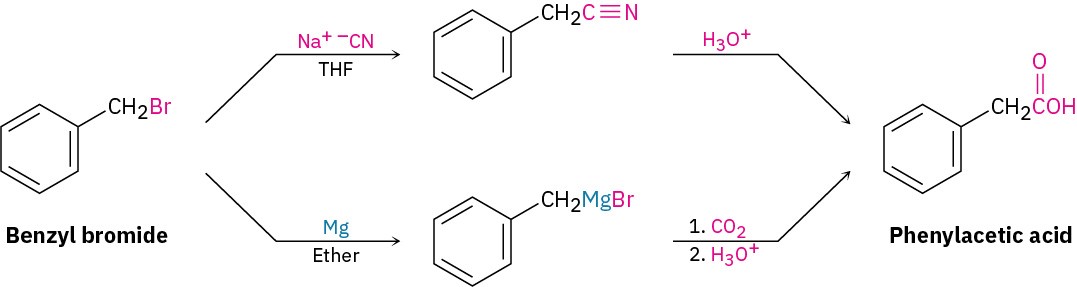
Worked Example 20.2Devising a Synthesis Route for a Carboxylic AcidHow would you prepare phenylacetic acid (PhCH2CO2H) from benzyl bromide (PhCH2Br)?StrategyWe’ve seen two methods for preparing carboxylic acids from alkyl halides: (1) cyanide ion displacement followed by hydrolysis and (2) formation of a Grignard reagent followed by carboxylation. The first method involves an SN2 reaction and is therefore limited to use with primary and some secondary alkyl halides. The second method involves formation of a Grignard reagent and is therefore limited to use with organic halides that have no acidic hydrogens or reactive functional groups elsewhere in the molecule. In the present instance, either method would work well.Solution
Problem 20-10
How would you prepare the following carboxylic acids? (a)
(CH3)3CCO2H from (CH3)3CCl
(b)
CH3CH2CH2CO2H from CH3CH2CH2Br

