12.1 Mass Spectrometry of Small Molecules: Magnetic-Sector Instruments
At its simplest, mass spectrometry (MS) is a technique for measuring the mass, and therefore the molecular weight (MW), of a molecule. In addition, it’s often possible to gain structural information about a molecule by measuring the masses of the fragments produced when molecules are broken apart.
More than 20 different kinds of commercial mass spectrometers are available depending on the intended application, but all have three basic parts: an ionization source in which sample molecules are given an electrical charge, a mass analyzer in which ions are separated by their mass-to-charge ratio, and a detector in which the separated ions are observed and counted.
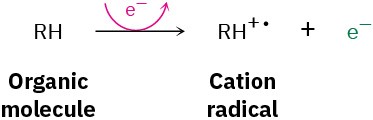
Among the most common mass spectrometers used for routine purposes in the laboratory is the electron-impact, magnetic-sector instrument shown schematically in Figure 12.2. A small amount of sample is vaporized into the ionization source, where it is bombarded by a stream of high-energy electrons. The energy of the electron beam can be varied but is commonly around 70 electron volts (eV), or 6700 kJ/mol. When a high-energy electron strikes an organic molecule, it dislodges a valence electron from the molecule, producing a cation radical—cation because the molecule has lost an electron and now has a positive charge; radical because the molecule now has an odd number of electrons.
Electron bombardment transfers so much energy that most of the cation radicals fragment after formation. They break apart into smaller pieces, some of which retain the positive charge and some of which are neutral. The fragments then flow through a curved pipe in a strong magnetic field, which deflects them into different paths according to their mass-to- charge ratio (m/z). Neutral fragments are not deflected by the magnetic field and are lost on the walls of the pipe, but positively charged fragments are sorted by the mass spectrometer onto a detector, which records them as peaks at the various m/z ratios. Since the number of charges z on each ion is usually 1, the value of m/z for each ion is simply its mass m. Masses up to approximately 2500 atomic mass units (amu) can be analyzed by this type of instrument.
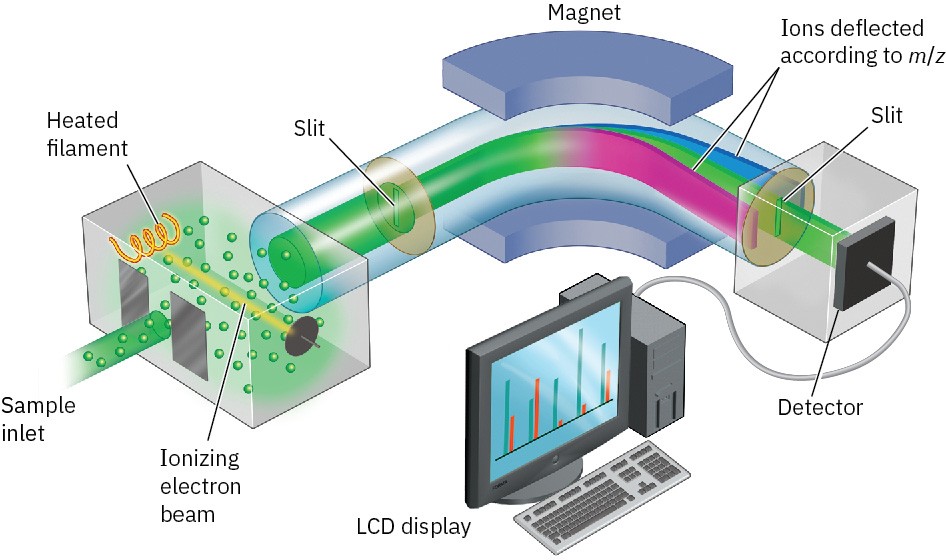
Figure 12.2 Representation of an electron-ionization, magnetic-sector mass spectrometer. Molecules are ionized by collision with high-energy electrons, causing some of the molecules to fragment. Passage of the charged fragments through a magnetic field then sorts them according to their mass.
Another common type of mass spectrometer uses what is called a quadrupole mass analyzer, which has a set of four solid rods is arranged parallel to the direction of the ion beam, with an oscillating electrostatic field is generated in the space between the rods. For a given field, only one m/z value will make it through the quadrupole region. The others will crash into the rods or the walls of the instrument and never reach the detector Figure 12.3.
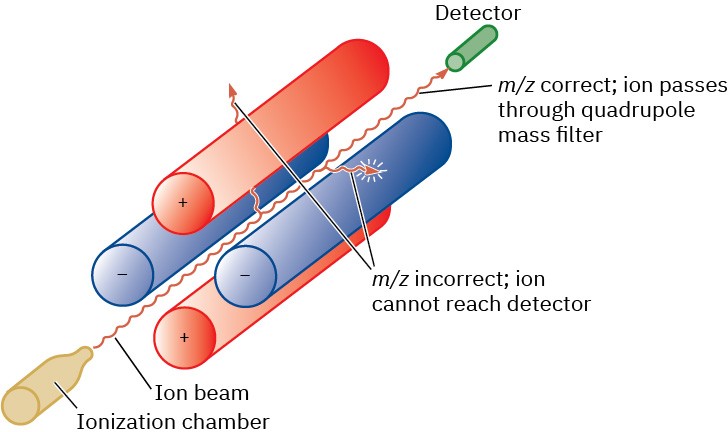
Figure 12.3 Representation of a quadrupole mass analyzer. Only ions of a certain m/z
will reach the detector; other ions will collide with the rods.
The mass spectrum of a compound is typically presented as a bar graph, with masses (m/z values) on the x axis and intensity, or relative abundance of ions of a given m/z striking the detector, on the y axis. The tallest peak, assigned an intensity of 100%, is called the base
peak, and the peak that corresponds to the unfragmented cation radical is called the parent peak, or the molecular ion (M+, or simply M). Figure 12.4 shows the mass spectrum of propane.
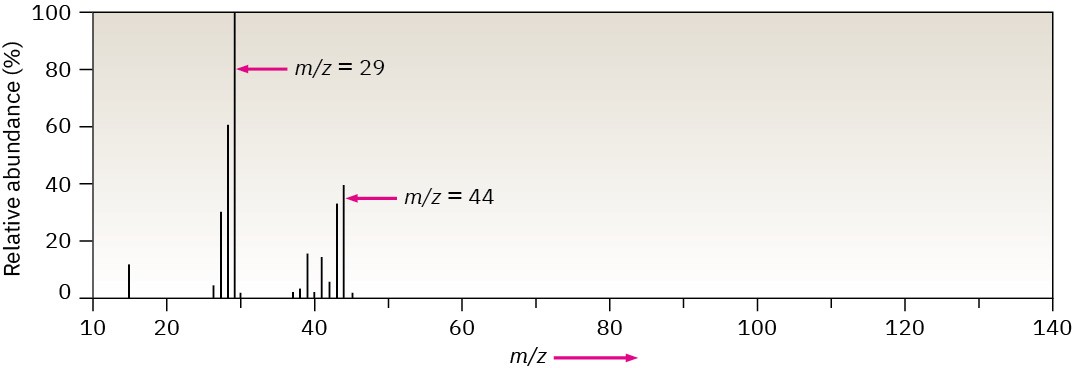
Figure 12.4Mass spectrum of propane (C3H8; MW = 44).
Mass spectral fragmentation patterns are usually complex, and the molecular ion is often not the base peak. The mass spectrum of propane in Figure 12.4, for instance, shows a molecular ion at m/z = 44 that is only about 30% as high as the base peak at m/z = 29. In addition, many other fragment ions are present.

