23.12 The Robinson Annulation Reaction
Carbonyl condensation reactions are perhaps the most versatile methods available for synthesizing complex molecules. By putting a few fundamental reactions together in the proper sequence, some remarkably useful transformations can be carried out. One such example is the Robinson annulation reaction for the synthesis of polycyclic molecules. The word annulation derives from the Latin annulus, meaning “ring,” so an annulation reaction is one that builds a new ring onto a molecule.
Named after Robert Robinson, a British chemist and 1947 Nobel Prize winner, the Robinson annulation is a two-step process that combines a Michael reaction with an intramolecular aldol reaction. It takes place between a nucleophilic donor, such as a β-keto ester; an enamine, or a β-diketone; and an α,β-unsaturated ketone acceptor, such as 3- buten-2-one. The product is a substituted 2-cyclohexenone.
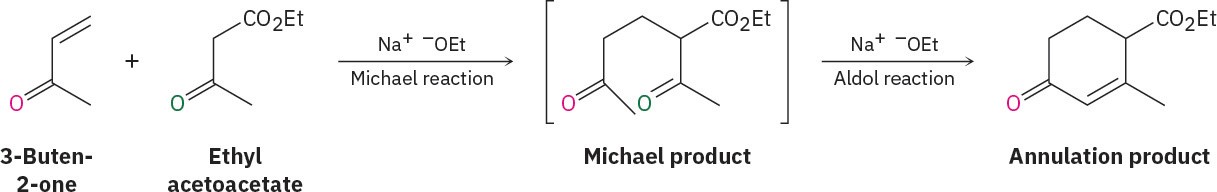
The first step of the Robinson annulation is simply a Michael reaction. An enamine or an enolate ion from a β-keto ester or β-diketone effects a conjugate addition to an α,β– unsaturated ketone, yielding a 1,5-diketone. But as we saw in Section 23.6, 1,5-diketones undergo intramolecular aldol condensation to yield cyclohexenones when treated with base. Thus, the final product contains a six-membered ring, and an annulation has been accomplished. One example of this occurs during a synthesis of the steroid hormone estrone (Figure 23.9).
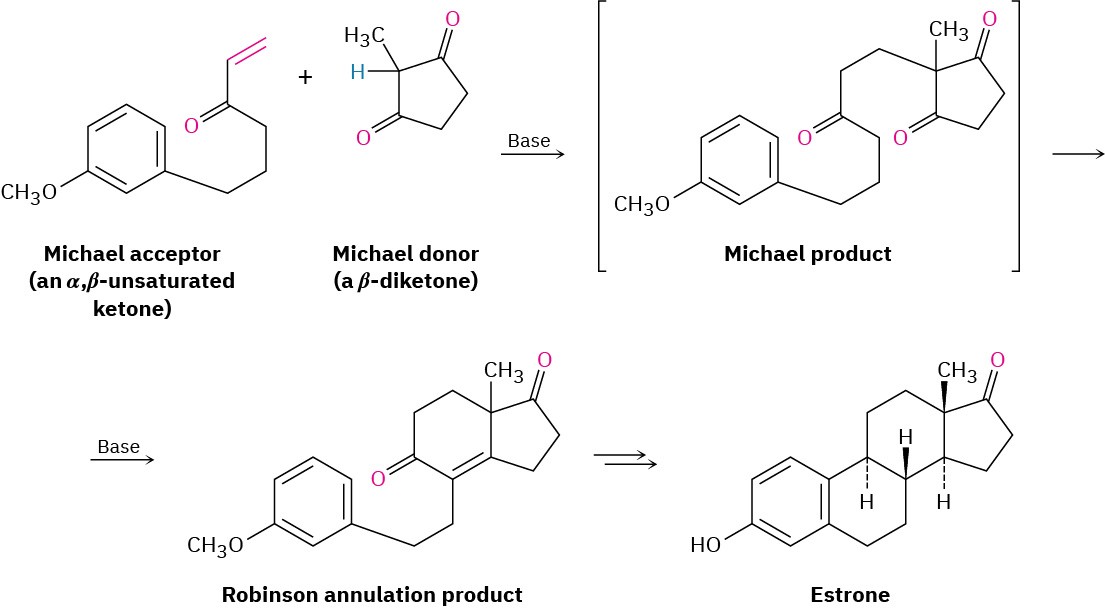
Figure 23.9 Synthesis of the steroid hormone estrone using a Robinson annulation reaction. The nucleophilic donor is a β-diketone.
In this example, the β-diketone 2-methyl-1,3-cyclopentanedione is used to generate the enolate ion required for Michael reaction and an aryl-substituted α,β-unsaturated ketone is used as the acceptor. Base-catalyzed Michael reaction between the two partners yields an intermediate triketone, which then cyclizes in an intramolecular aldol condensation to give a Robinson annulation product. Several further transformations are required to complete the synthesis of estrone.
Problem 23-21
What product would you expect from a Robinson annulation reaction of 2-methyl-1,3- cyclopentanedione with 3-buten-2-one?
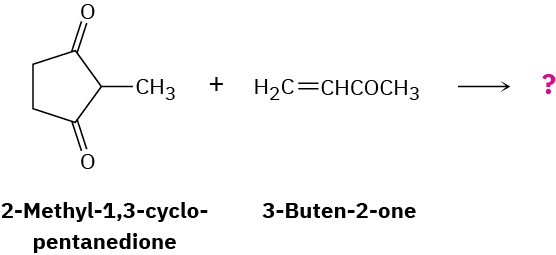
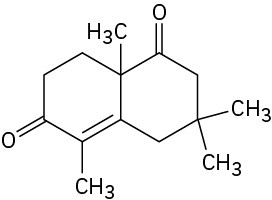
Problem 23-22
How would you prepare the following compound using a Robinson annulation reaction between a β-diketone and an α,β-unsaturated ketone? Draw the structures of both reactants and the intermediate Michael addition product.