1.8 sp2 Hybrid Orbitals and the Structure of Ethylene
The bonds we’ve seen in methane and ethane are called single bonds because they result from the sharing of one electron pair between bonded atoms. It was recognized nearly 150 years ago, however, that carbon atoms can also form double bonds by sharing two electron pairs between atoms or triple bonds by sharing three electron pairs. Ethylene, for instance, has the structure H!C=CH! and contains a carbon–carbon double bond, while acetylene has the structure HC≡CH and contains a carbon–carbon triple bond.
How are multiple bonds described by valence bond theory? When we discussed sp3 hybrid orbitals in Section 1.6, we said that the four valence-shell atomic orbitals of carbon combine to form four equivalent sp3 hybrids. Imagine instead that the 2s orbital combines with only two of the three available 2p orbitals. Three sp2 hybrid orbitals result, and one 2p orbital remains unchanged. Like sp3 hybrids, sp2 hybrid orbitals are unsymmetrical about the nucleus and are strongly oriented in a specific direction so they can form strong bonds. The three sp2 orbitals lie in a plane at angles of 120° to one another, with the remaining p orbital perpendicular to the sp2 plane, as shown in Figure 1.14.
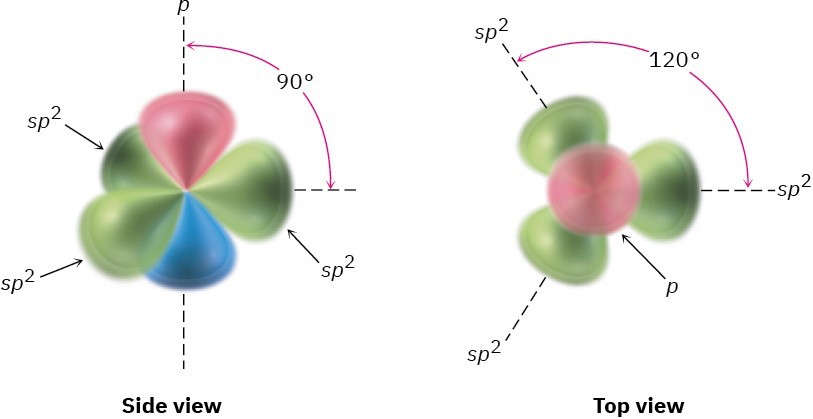
Figure 1.14 sp2 Hybridization. The three equivalent sp2 hybrid orbitals lie in a plane at angles of 120° to one another, and a single unhybridized p orbital (red/blue) is perpendicular to the sp2 plane.
When two carbons with sp2 hybridization approach each other, they form a strong σ bond by sp2–sp2 head-on overlap. At the same time, the unhybridized p orbitals interact by sideways overlap to form what is called a pi (π) bond. The combination of an sp2–sp2 σ bond and a 2p–2p π bond results in the sharing of four electrons and the formation of a carbon–carbon double bond (Figure 1.15). Note that the electrons in the σ bond occupy the region centered between nuclei, while the electrons in the π bond occupy regions above and below a line drawn between nuclei.
To complete the structure of ethylene, four hydrogen atoms form σ bonds with the remaining four sp2 orbitals. Ethylene thus has a planar structure, with H–C–H and H–C–C bond angles of approximately 120°. (The actual values are 117.4° for the H–C–H bond angle
and 121.3° for the H–C–C bond angle.) Each C–H bond has a length of 108.7 pm and a strength of 464 kJ/mol (111 kcal/mol).
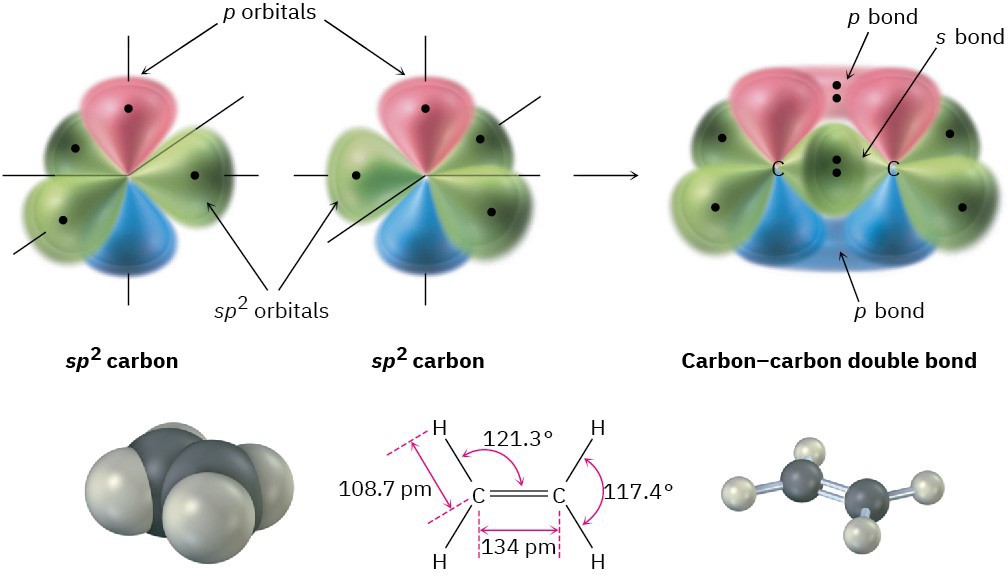
Figure 1.15 The structure of ethylene. One part of the double bond in ethylene results from σ (head-on) overlap of sp2 hybrid orbitals, and the other part results from π (sideways) overlap of unhybridized p orbitals (red/blue). The π bond has regions of electron density above and below a line drawn between nuclei.
As you might expect, the carbon–carbon double bond in ethylene is both shorter and stronger than the single bond in ethane because it has four electrons bonding the nuclei together rather than two. Ethylene has a C=C bond length of 134 pm and a strength of 728 kJ/mol (174 kcal/mol) versus a C–C length of 153 pm and a strength of 377 kJ/mol for ethane. The carbon–carbon double bond is less than twice as strong as a single bond because the sideways overlap in the π part of the double bond is not as great as the head-on overlap in the σ part.
Worked Example 1.3Drawing Electron-Dot and Line-Bond StructuresCommonly used in biology as a tissue preservative, formaldehyde, CH2O, contains a carbon–oxygen double bond. Draw electron-dot and line-bond structures of formaldehyde, and indicate the hybridization of the carbon orbitals.StrategyWe know that hydrogen forms one covalent bond, carbon forms four, and oxygen forms two. Trial and error, combined with intuition, is needed to fit the atoms together.SolutionThere is only one way that two hydrogens, one carbon, and one oxygen can combine:
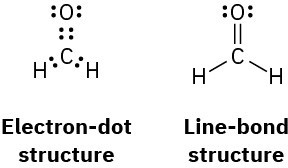
Like the carbon atoms in ethylene, the carbon atom in formaldehyde is in a double bond and its orbitals are therefore sp2-hybridized.
Problem 1-10
Draw a line-bond structure for propene, CH3CH=CH2. Indicate the hybridization of the orbitals on each carbon, and predict the value of each bond angle.
Problem 1-11
Draw a line-bond structure for 1,3-butadiene, H2C=CH–CH=CH2. Indicate the hybridization of the orbitals on each carbon, and predict the value of each bond angle.
Problem 1-12
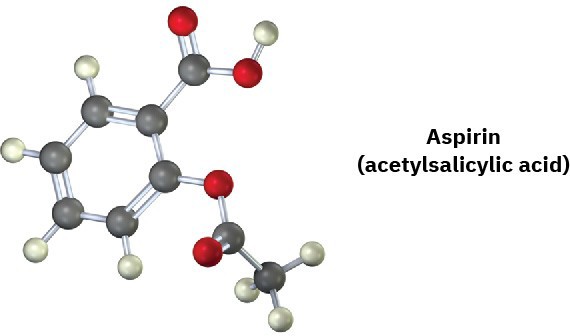
A molecular model of aspirin (acetylsalicylic acid) is shown. Identify the hybridization of the orbitals on each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (black = C, red = O, gray = H).