17.1 Naming Alcohols and Phenols
Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of organic groups bonded to the hydroxyl-bearing carbon.
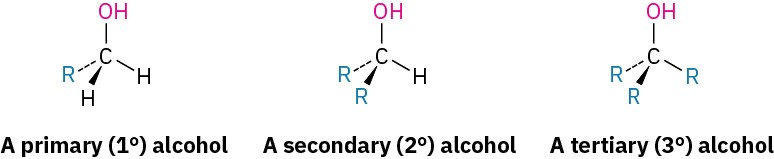
Simple alcohols are named in the IUPAC system as derivatives of the parent alkane, using the suffix –ol.
RULE 1
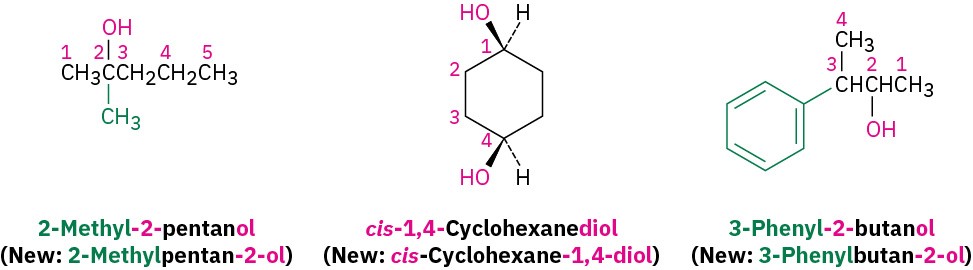
Select the longest carbon chain containing the hydroxyl group, and derive the parent name by replacing the –e ending of the corresponding alkane with –ol. The –e is deleted to prevent the occurrence of two adjacent vowels: propanol rather than propaneol, for example.
RULE 2
Number the alkane chain beginning at the end nearer the hydroxyl group.
RULE 3
Number the substituents according to their position on the chain, and write the name, listing the substituents in alphabetical order and identifying the position to which the –OH is bonded. Note that in naming cis-1,4-cyclohexanediol, the final -e of cyclohexane is not deleted because the next letter, d, is not a vowel; that is, cyclohexanediol rather than cyclohexandiol. Also, as with alkenes (Section 7.3), newer IUPAC naming recommendations place the locant immediately before the suffix rather than before the parent.
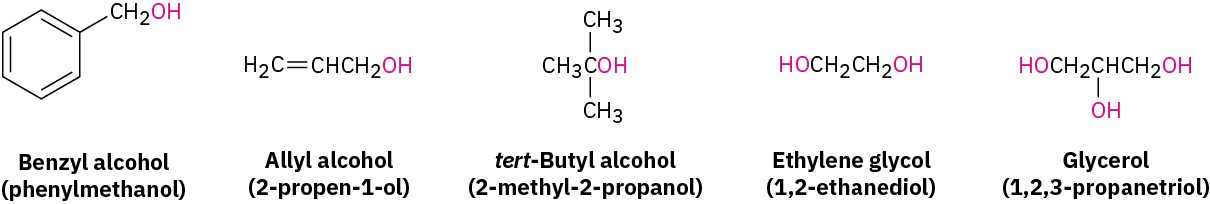
Some simple and widely occurring alcohols have common names that are accepted by IUPAC. For example:
Phenols are named as described previously for aromatic compounds according to the rules discussed in Section 15.1, with –phenol used as the parent name rather than –benzene.
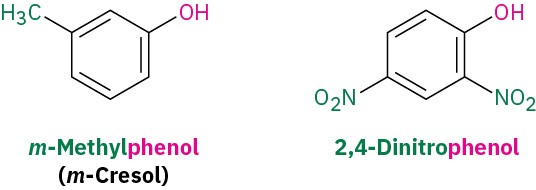
Problem 17-1
Give IUPAC names for the following compounds: (a)
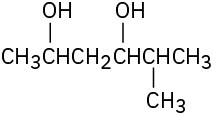
(b)
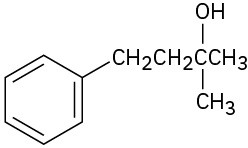
(c)
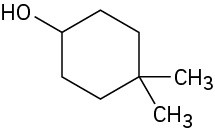
(d)
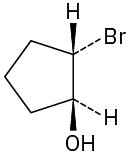
(e)
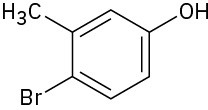
(f)
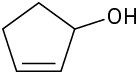
Problem 17-2
Draw structures corresponding to the following IUPAC names: (a)
(Z)-2-Ethyl-2-buten-1-ol (b)
3-Cyclohexen-1-ol (c)
trans-3-Chlorocycloheptanol (d)
1,4-Pentanediol (e)
2,6-Dimethylphenol (f)
o-(2-Hydroxyethyl)phenol

