29.7 The Citric Acid Cycle
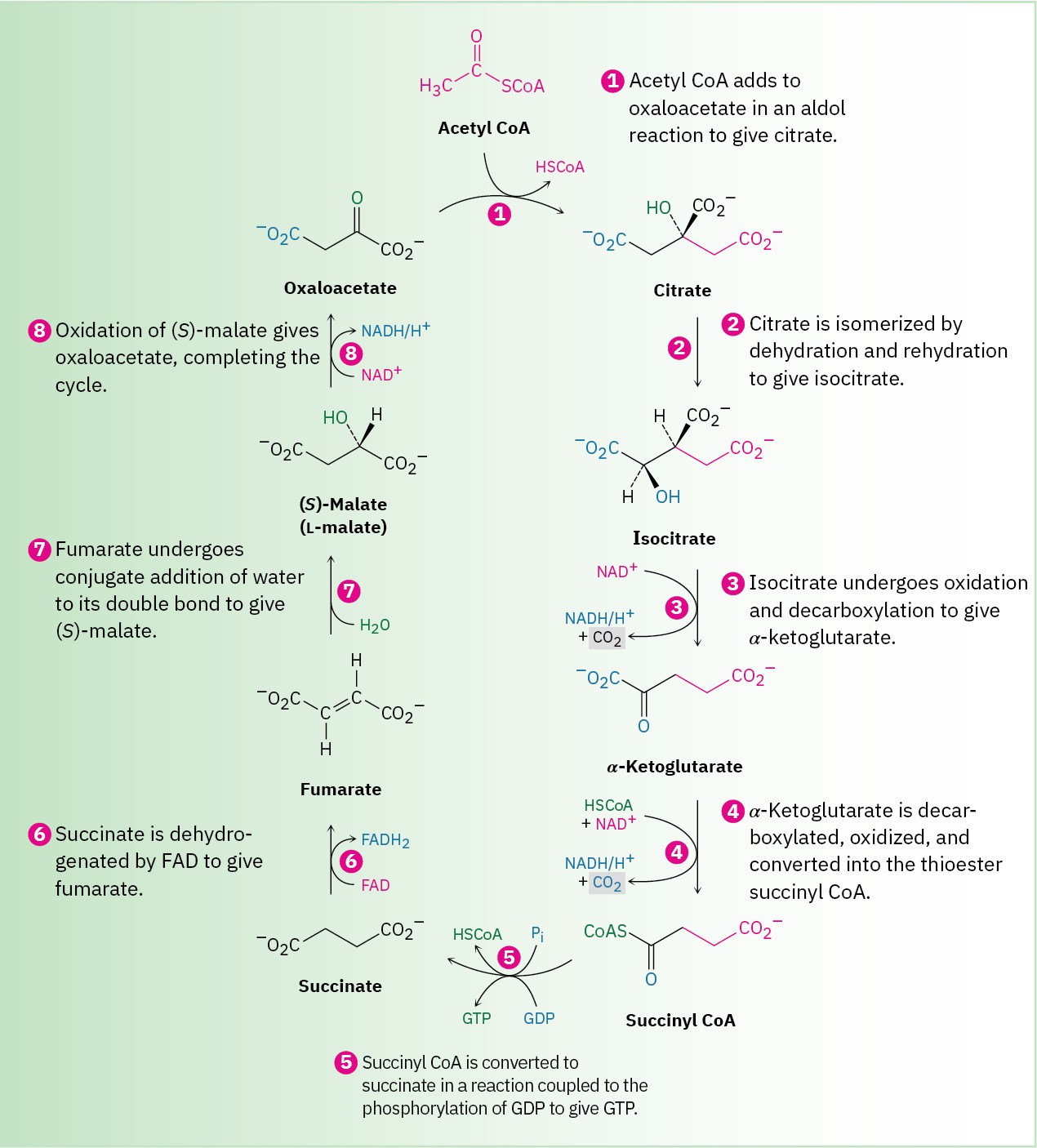
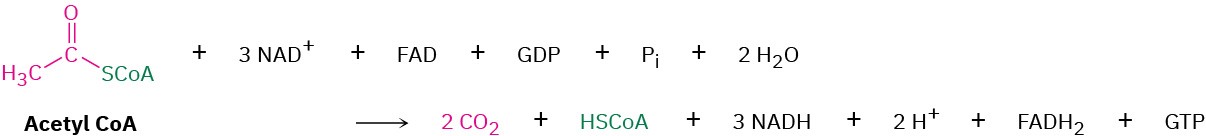
The initial stages of catabolism result in the conversion of both fats and carbohydrates into acetyl groups that are bonded through a thioester link to coenzyme A. Acetyl CoA then enters the next stage of catabolism—the citric acid cycle, also called the tricarboxylic acid (TCA) cycle, or Krebs cycle, after Hans Krebs, who unraveled its complexities in 1937. The overall result of the cycle is the conversion of an acetyl group into two molecules of CO2 plus reduced coenzymes by the eight-step reaction sequence shown in Figure 29.14.
As its name implies, the citric acid cycle is a closed loop of reactions in which the product of the final step (oxaloacetate) is a reactant in the first step. The intermediates are constantly regenerated, flowing continuously through the cycle, which operates as long as the oxidizing coenzymes NAD+ and FAD are available. To meet this condition, the reduced coenzymes NADH and FADH2 must be reoxidized via the electron-transport chain, which in turn relies on oxygen as the ultimate electron acceptor. Thus, the cycle is dependent on the availability of oxygen and on the operation of the electron-transport chain.
Figure 29.14 MECHANISM
The citric acid cycle is an eight-step series of reactions that results in the conversion of an acetyl group into two molecules of CO2 plus reduced coenzymes. Individual steps are explained in the text.
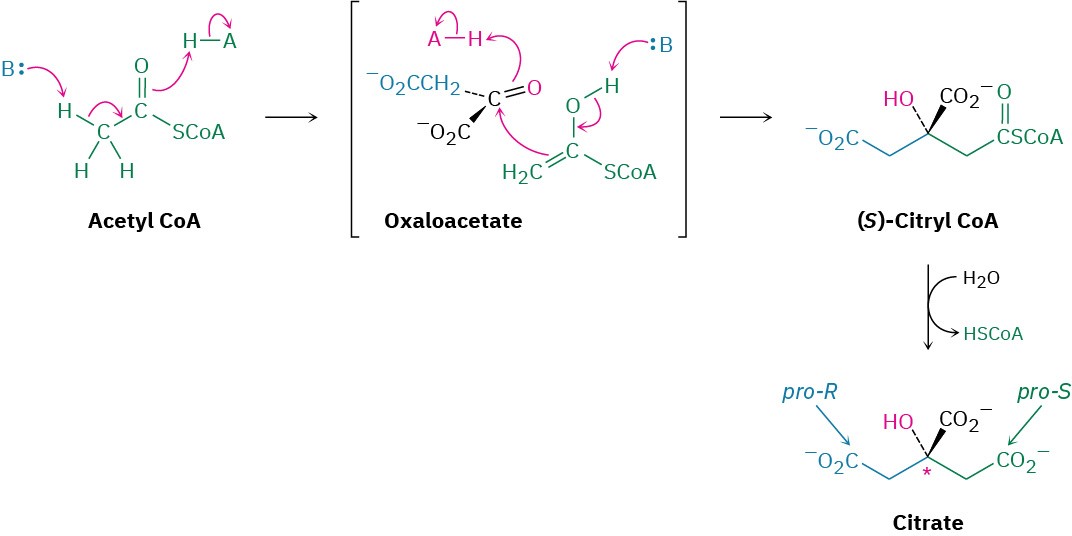
Step 1 of Figure 29.14: Addition to Oxaloacetate
Acetyl CoA enters the citric acid cycle in step 1 by nucleophilic addition to the oxaloacetate carbonyl group, to give (S)-citryl CoA. This addition is an aldol reaction and is catalyzed by citrate synthase, as discussed in Section 26.11. (S)-Citryl CoA is then hydrolyzed to citrate by a typical nucleophilic acyl substitution reaction with water, catalyzed by the same citrate synthase enzyme.
Note that the hydroxyl-bearing carbon of citrate is a prochirality center and contains two identical arms. Because the initial aldol reaction of acetyl CoA to oxaloacetate occurs specifically from the Si face of the ketone carbonyl group, the pro-S arm of citrate is derived from acetyl CoA and the pro-R arm is derived from oxaloacetate.
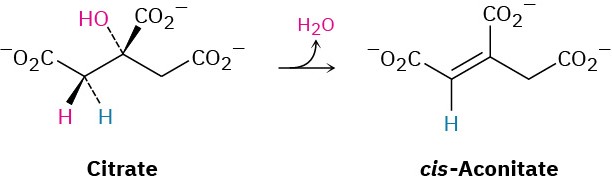
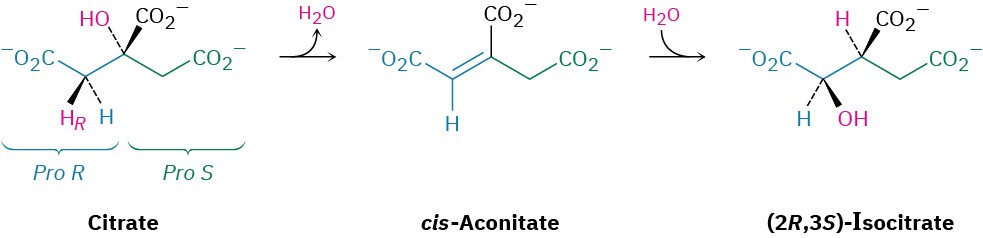
Step 2 of Figure 29.14: Isomerization
Citrate, a prochiral tertiary alcohol, is next converted into its isomer, (2R,3S)-isocitrate, a chiral secondary alcohol. The isomerization occurs in two steps, both of which are catalyzed by the same aconitase enzyme. The initial step is an E1cB dehydration of a β– hydroxy acid to give cis-aconitate, the same sort of reaction that occurs in step 9 of glycolysis (Figure 29.8). The second step is a conjugate nucleophilic addition of water to the C═C bond (Section 19.13). The dehydration of citrate takes place specifically on the
pro-R arm—the one derived from oxaloacetate—rather than on the pro-S arm derived from acetyl CoA.
Step 3 of Figure 29.14: Oxidation and Decarboxylation
(2R,3S)-Isocitrate, a secondary alcohol, is oxidized by NAD+ in step 3 to give the ketone oxalosuccinate, which loses CO2 to give α-ketoglutarate. Catalyzed by isocitrate dehydrogenase, the decarboxylation is a typical reaction of a β-keto acid, just like that in the acetoacetic ester synthesis (Section 22.7). The enzyme requires a divalent cation as a cofactor to polarize the ketone carbonyl group and make it a better electron acceptor.
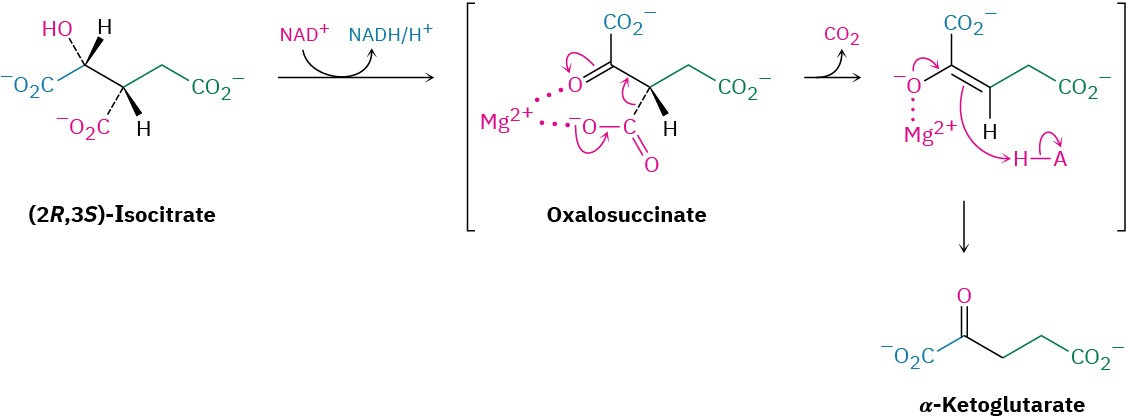
Step 4 of Figure 29.14: Oxidative Decarboxylation
The transformation of α-ketoglutarate to succinyl CoA in step 4 is a multistep process just like the transformation of pyruvate to acetyl CoA that we saw in Figure 29.13. In both cases, an α-keto acid loses CO2 and is oxidized to a thioester in a series of steps catalyzed by a multienzyme dehydrogenase complex. As in the conversion of pyruvate to acetyl CoA, the reaction involves an initial nucleophilic addition reaction of thiamin diphosphate ylide to α– ketoglutarate, followed by decarboxylation. Reaction with lipoamide, elimination of TPP ylide, and finally a transesterification of the dihydrolipoamide thioester with coenzyme A yields succinyl CoA.
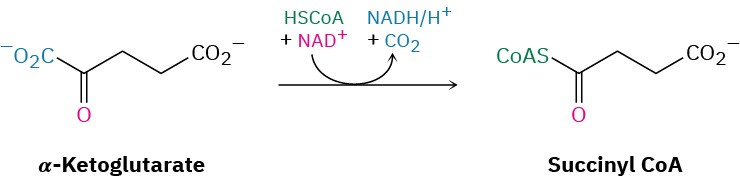
Step 5 of Figure 29.14: Acyl CoA Cleavage
Succinyl CoA is converted to succinate in step 5. This reaction is catalyzed by succinyl CoA synthetase and is coupled with phosphorylation of guanosine diphosphate (GDP) to give guanosine triphosphate (GTP). The overall transformation is similar to that of steps 6 through 8 in glycolysis (Figure 29.8), in which a thioester is converted into an acyl phosphate and a phosphate group is then transferred to ADP. The overall result is a “hydrolysis” of the thioester group without involvement of water.
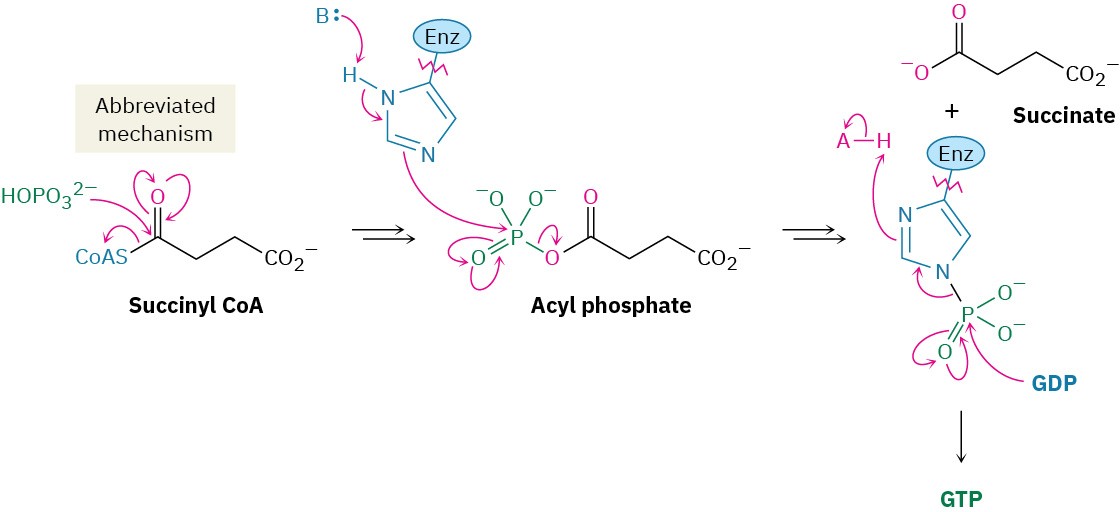
Step 6 of Figure 29.14: Dehydrogenation
Succinate is dehydrogenated in step 6 by the FAD-dependent succinate dehydrogenase to give fumarate. This process is analogous to what occurs during the β-oxidation pathway of fatty-acid catabolism (Section 29.3). The reaction is stereospecific, removing the pro-S hydrogen from one carbon and the pro-R hydrogen from the other.
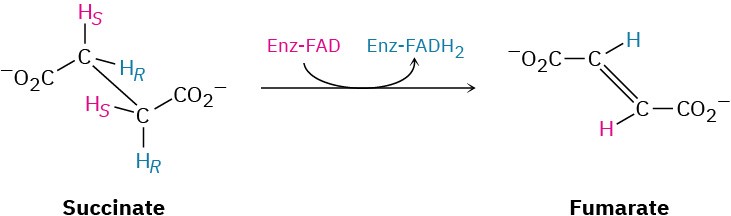
Steps 7–8 of Figure 29.14: Hydration and Oxidation
The final two steps in the citric acid cycle are the conjugate nucleophilic addition of water to fumarate to yield (S)-malate and the oxidation of (S)-malate by NAD+ to give oxaloacetate. The addition is catalyzed by fumarase and is mechanistically similar to the addition of water to cis-aconitate in step 2. This reaction occurs through an enolate-ion intermediate, which is protonated on the side opposite the OH, leading to a net anti addition.
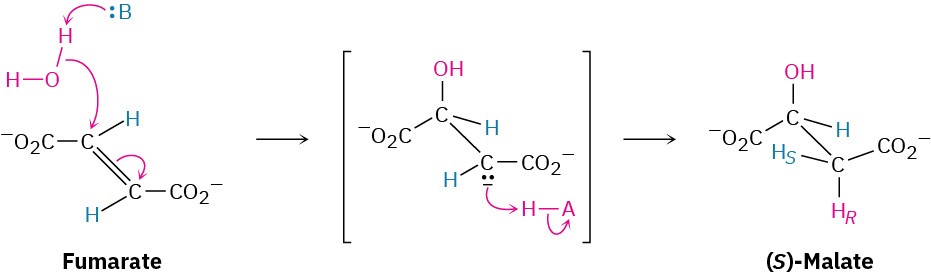
The final step is the oxidation of (S)-malate by NAD+ to give oxaloacetate, a reaction catalyzed by malate dehydrogenase. The citric acid cycle has now returned to its starting point, ready to revolve again. The overall result of the cycle is
Problem 29-10
Which of the substances in the citric acid cycle are tricarboxylic acids, thus giving the cycle its alternative name?
Problem 29-11
Write mechanisms for step 2 of the citric acid cycle, the dehydration of citrate and the addition of water to aconitate.
Problem 29-12
Is the pro-R or pro-S hydrogen removed from citrate during the dehydration in step 2 of the citric acid cycle? Does the elimination reaction occur with syn or anti geometry?