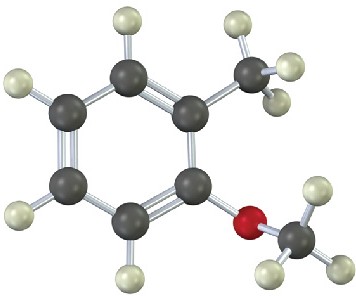
16.5 Trisubstituted Benzenes: Additivity of Effects
Electrophilic substitution of a disubstituted benzene ring is governed by the same resonance and inductive effects that influence monosubstituted rings. The only difference is that it’s necessary to consider the additive effects of two different groups. In practice, this isn’t as difficult as it sounds; three rules are usually sufficient.
RULE 1
If the directing effects of the two groups reinforce each other, the situation is straightforward. In p-nitrotoluene, for example, both the methyl and the nitro group direct further substitution to the same position (ortho to the methyl = meta to the nitro). A single product is thus formed on electrophilic substitution.
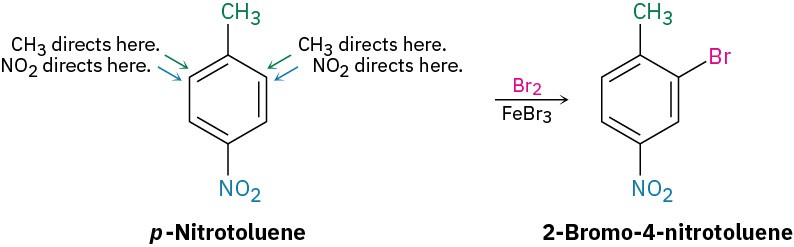
RULE 2
If the directing effects of the two groups oppose each other, the more powerful activating group has the dominant influence, but mixtures of products are often formed. For example, bromination of p-methylphenol yields primarily 2-bromo-4-methylphenol because –OH is a more powerful activator than –CH3.
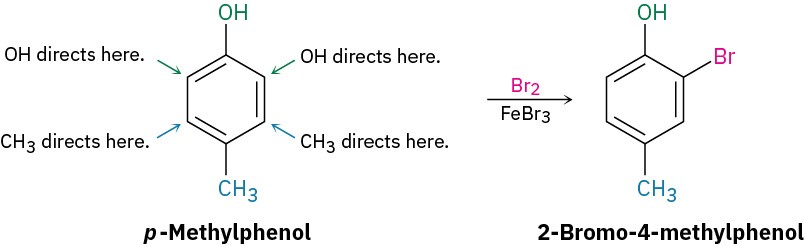
RULE 3
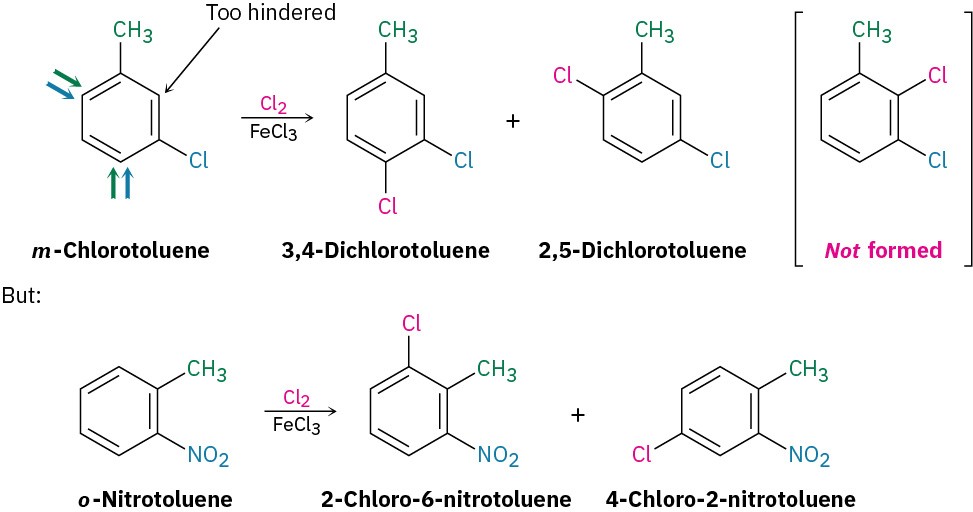
Further substitution rarely occurs between the two groups in a meta-disubstituted compound because this site is too hindered. Aromatic rings with three adjacent substituents must therefore be prepared by some other route, such as by substitution of an ortho-disubstituted compound.
Worked Example 16.3Predicting the Product of Substitution on a Disubstituted BenzeneWhat product would you expect from bromination of p-methylbenzoic acid?StrategyIdentify the two substituents present on the ring, decide the directing effect of each and, if necessary, decide which substituent is the stronger activator. In the present case, the carboxyl group (–CO2H) is a meta director and the methyl group is an ortho and para director. Both groups direct bromination to the position next to the methyl group, yielding 3-bromo-4-methylbenzoic acid.Solution
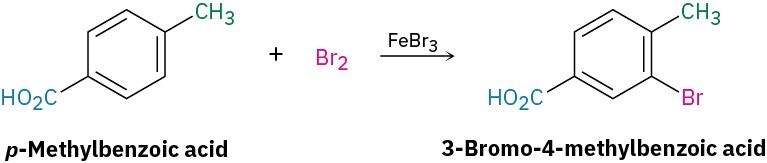
Problem 16-14
At what position would you expect electrophilic substitution to occur in each of the following substances:
(a)
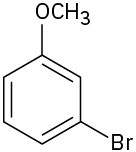
(b)
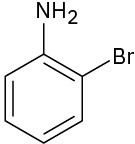
(c)
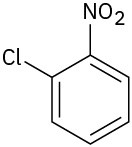
Problem 16-15
Show the major product(s) from reaction of the following substances with (1) CH3CH2Cl, AlCl3 and (2) HNO3, H2SO4:
(a)
(b)