30.6 Stereochemistry of Cycloadditions
How can we predict whether a given cycloaddition reaction will occur with suprafacial or with antarafacial geometry? According to frontier orbital theory, a cycloaddition reaction takes place when a bonding interaction occurs between the HOMO of one reactant and the LUMO of the other. An intuitive explanation of this rule is to imagine that one reactant donates electrons to the other. As with electrocyclic reactions, it’s the electrons in the HOMO of the first reactant that are least tightly held and most likely to be donated. Of course when the second reactant accepts those electrons, they must go into a vacant, unoccupied orbital—the LUMO.
For a [4 + 2] cycloaddition (Diels–Alder reaction), let’s arbitrarily select the diene LUMO and the alkene HOMO. The symmetries of the two ground-state orbitals are such that bonding of the terminal lobes can occur with suprafacial geometry (Figure 30.10), so the Diels–Alder reaction takes place readily under thermal conditions. Note that, as with electrocyclic reactions, we need be concerned only with the terminal lobes. For purposes of prediction, interactions among the interior lobes need not be considered.
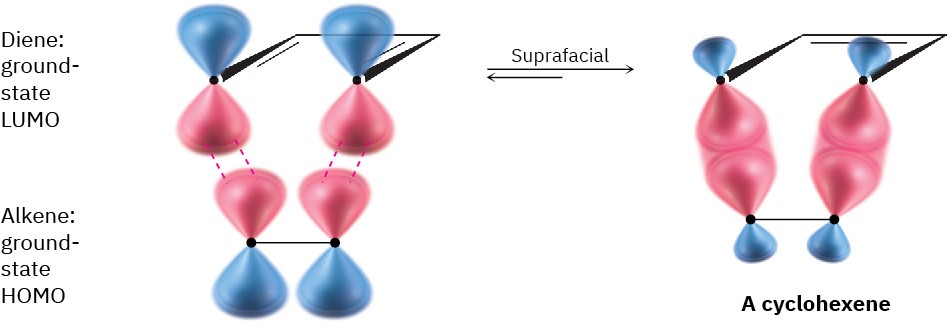
Figure 30.10Interaction of diene LUMO and alkene HOMO in a suprafacial [4 + 2] cycloaddition reaction (Diels–Alder reaction).
In contrast with the thermal [4 + 2] Diels–Alder reaction, the [2 + 2] cycloaddition of two alkenes to yield a cyclobutane can only occur photochemically. The explanation for this follows from orbital-symmetry arguments. Looking at the ground-state HOMO of one alkene and the LUMO of the second alkene, it’s apparent that a thermal [2 + 2] cycloaddition must take place by an antarafacial pathway (Figure 30.11a). Geometric constraints make the antarafacial transition state difficult, however, and so concerted thermal [2 + 2] cycloadditions are not observed.
In contrast with the thermal process, photochemical [2 + 2] cycloadditions are observed. Irradiation of an alkene with UV light excites an electron from ψ1, the ground-state HOMO, to ψ2*, which becomes the excited-state HOMO. Interaction between the excited-state HOMO of one alkene and the LUMO of the second alkene allows a photochemical [2 + 2] cycloaddition reaction to occur by a suprafacial pathway (Figure 30.11b).
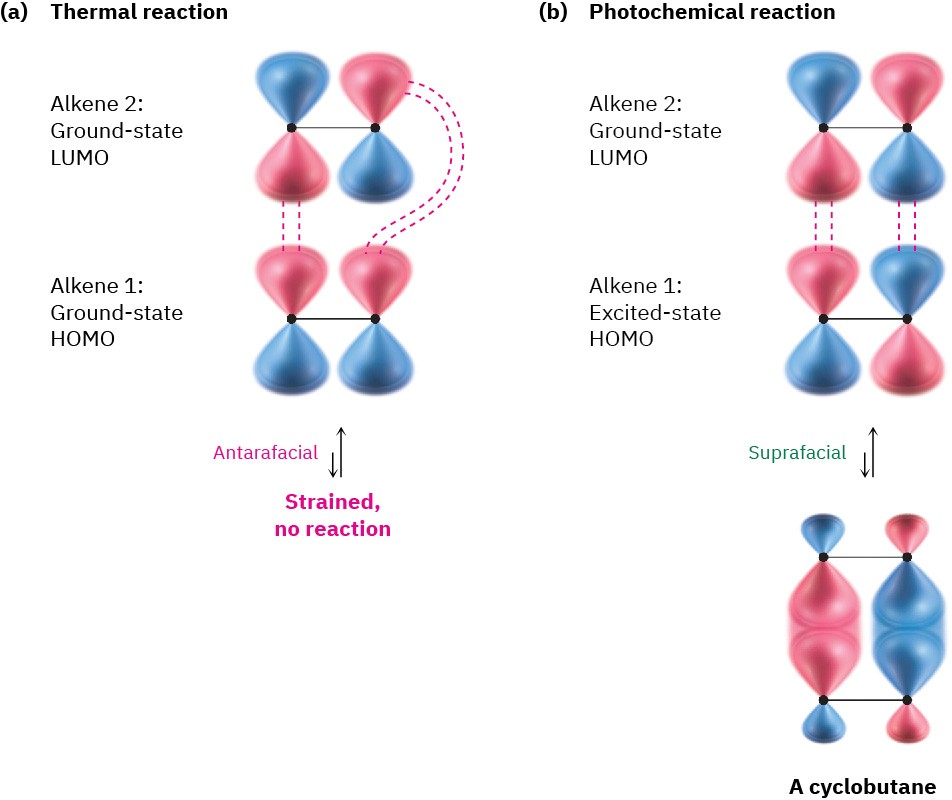
Figure 30.11 (a) Interaction of a ground-state HOMO and a ground-state LUMO in a potential [2 + 2] cycloaddition does not occur thermally because the antarafacial geometry is too strained. (b) Interaction of an excited-state HOMO and a ground- state LUMO in a photochemical [2 + 2] cycloaddition reaction is less strained, however, and occurs with suprafacial geometry.
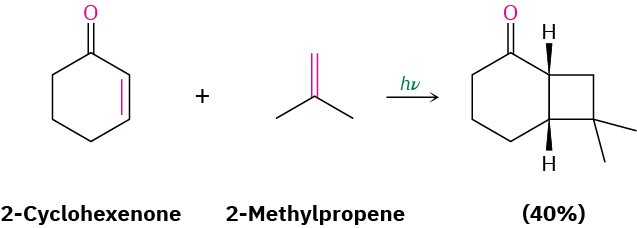
The photochemical [2 + 2] cycloaddition reaction occurs smoothly, particularly with α,β– unsaturated carbonyl compounds, and represents one of the best methods known for synthesizing cyclobutane rings. For example:
Thermal and photochemical cycloaddition reactions always take place with opposite stereochemistry. As with electrocyclic reactions, we can categorize cycloadditions according to the total number of electron pairs (double bonds) involved in the rearrangement. Thus, a thermal [4 + 2] Diels–Alder reaction between a diene and a dienophile involves an odd number (three) of electron pairs and takes place by a suprafacial pathway. A thermal [2 + 2] reaction between two alkenes involves an even number (two) of electron pairs and must take place by an antarafacial pathway. For
photochemical cyclizations, these selectivities are reversed. These general rules are given in Table 30.2.
Table 30.2 Stereochemical Rules for Cycloaddition Reactions
|
Thermal reaction |
Photochemical reaction |
|
|
Even number |
Antarafacial |
Suprafacial |
|
Odd number |
Suprafacial |
Antarafacial |
Problem 30-5
What stereochemistry would you expect for the product of the Diels–Alder reaction between (2E,4E)-2,4-hexadiene and ethylene? What stereochemistry would you expect if (2E,4Z)-2,4-hexadiene were used instead?
Problem 30-6
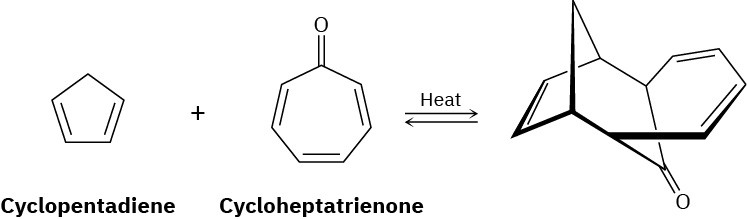
1,3-Cyclopentadiene reacts with cycloheptatrienone to give the product shown. Tell what kind of reaction is involved, and explain the observed result. Is the reaction suprafacial or antarafacial?