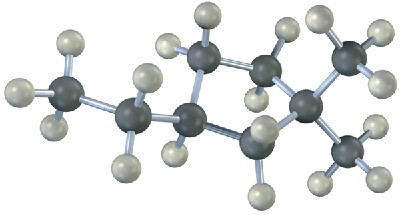
4.1 Naming Cycloalkanes
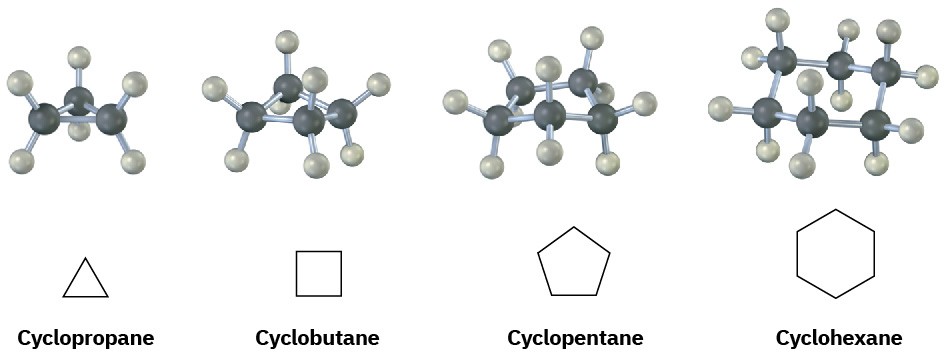
Saturated cyclic hydrocarbons are called cycloalkanes, or alicyclic compounds (aliphatic cyclic). Because cycloalkanes consist of rings of −CH2−units, they have the general formula (CH2)n, or CnH2n, and can be represented by polygons in skeletal drawings.
Substituted cycloalkanes are named by rules similar to those we saw in (Section 3.4) for open-chain alkanes. For most compounds, there are only two steps.
STEP 1
Find the parent.
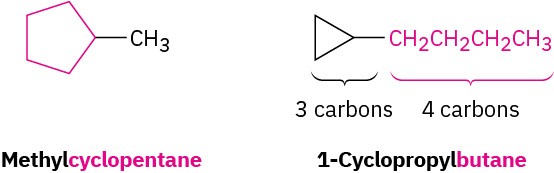
Count the number of carbon atoms in the ring and the number in the largest substituent. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane. If the number of carbon atoms in the largest substituent is greater than the number in the ring, the compound is named as a cycloalkyl-substituted alkane. For example:
STEP 2
Number the substituents, and write the name.
For an alkyl- or halo-substituted cycloalkane, choose a point of attachment as carbon 1 and number the substituents on the ring so that the second substituent has as low a number as possible. If ambiguity still exists, number so that the third or fourth substituent has as low a number as possible, until a point of difference is found.
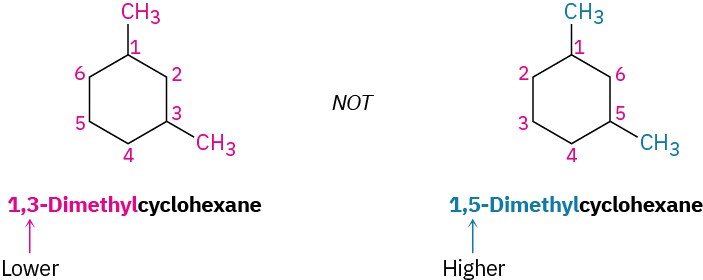
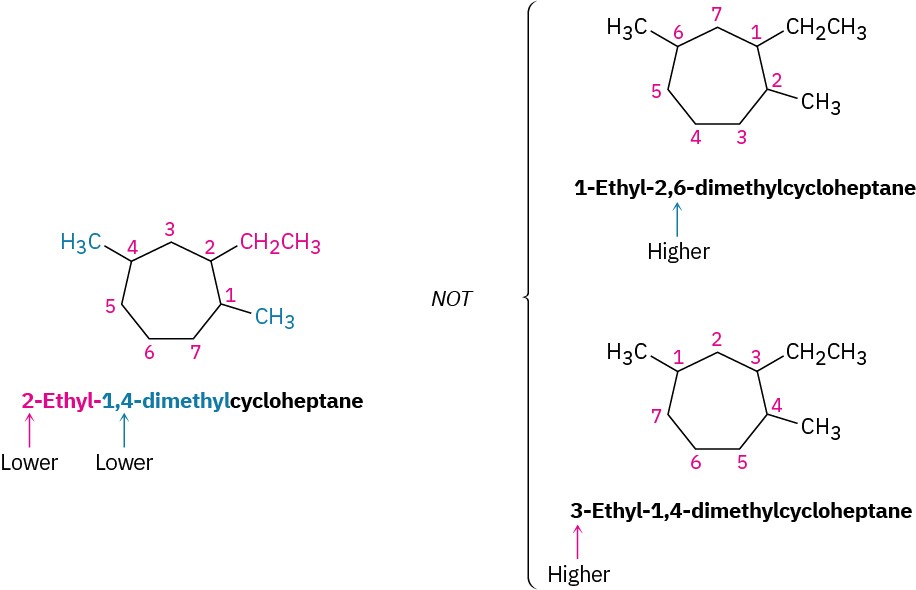
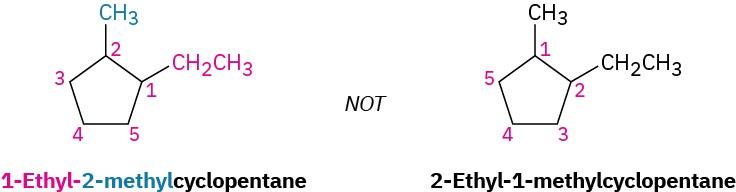
- When two or more different alkyl groups are present that could potentially take the same numbers, number them by alphabetical priority, ignoring numerical prefixes such as di- and tri-.
- If halogens are present, treat them just like alkyl groups.
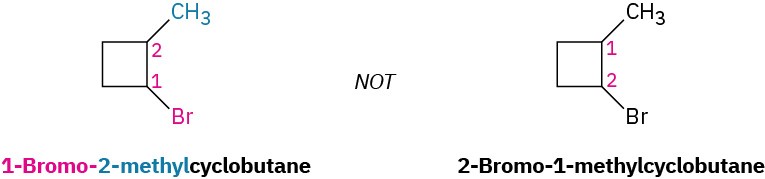
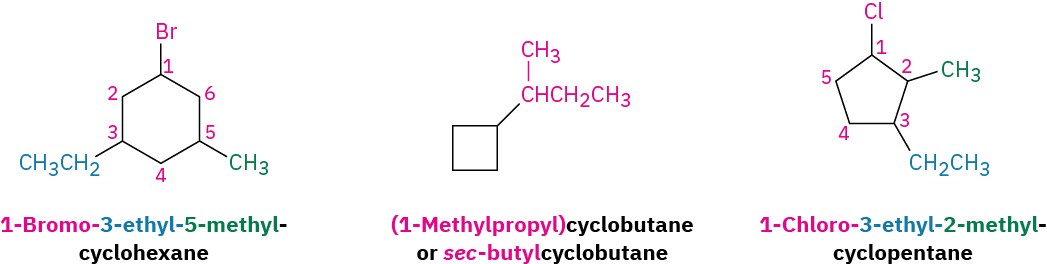
Some additional examples follow:
Problem 4-1
Give IUPAC names for the following cycloalkanes: (a)
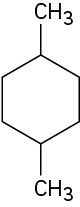
(b)
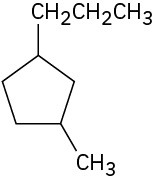
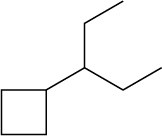
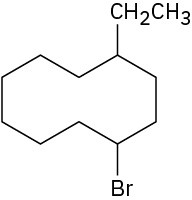
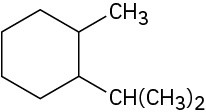
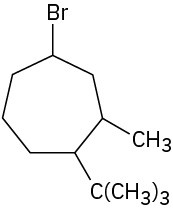
Problem 4-2
Draw structures corresponding to the following IUPAC names: (a)
1,1-Dimethylcyclooctane (b)
3-Cyclobutylhexane (c)
1,2-Dichlorocyclopentane (d)
1,3-Dibromo-5-methylcyclohexane Problem 4-3
Name the following cycloalkane: