26.7 Peptide Synthesis
Once the structure of a peptide is known, its synthesis can be undertaken—perhaps to obtain a larger amount for biological evaluation. A simple amide might be formed by treating an amine and a carboxylic acid with a carbodiimide (either DCC or EDC; Section 21.3), but peptide synthesis is a more difficult problem because many different amide bonds must be formed in a specific order, rather than at random.
The solution to the specificity problem is protection (Section 17.8). If we want to couple alanine with leucine to synthesize Ala-Leu, for instance, we could protect the –NH2 group of alanine and the –CO2H group of leucine to shield them from reacting, then form the desired Ala-Leu amide bond by reaction with EDC or DCC, and then remove the protecting groups.
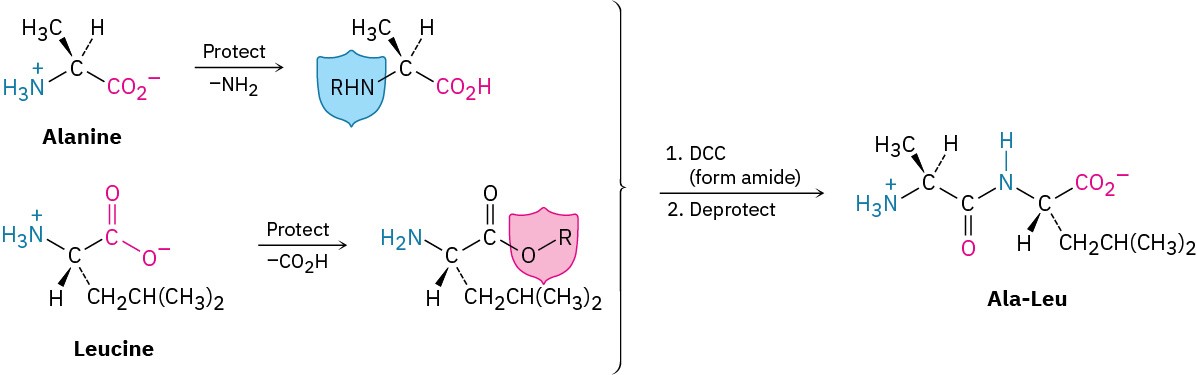
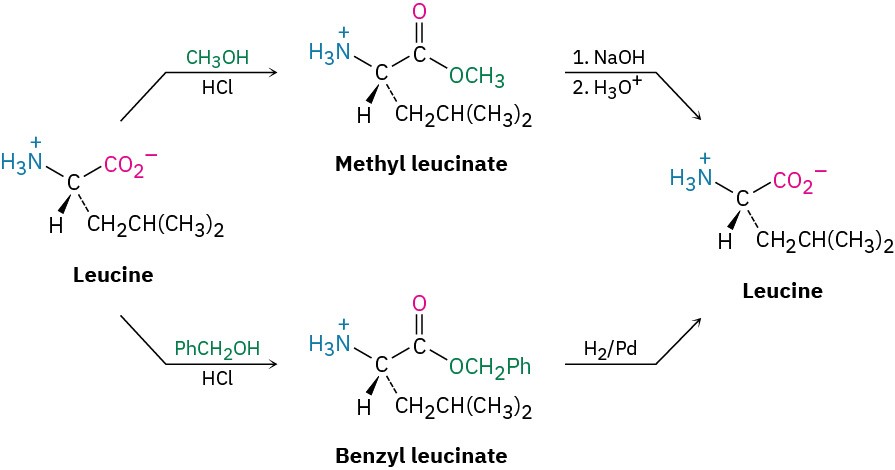
A number of different amino- and carboxyl-protecting groups have been devised, but only a few are used in peptide synthesis. Carboxyl groups are often protected simply by converting them into methyl or benzyl esters. Both groups are easily introduced by standard methods of ester formation (Section 21.6) and are easily removed by mild hydrolysis with aqueous NaOH. Benzyl esters can also be cleaved by catalytic hydrogenolysis of the weak benzylic C–O bond (RCO!–CH!Ph + H! → RCO!H + PhCH“).
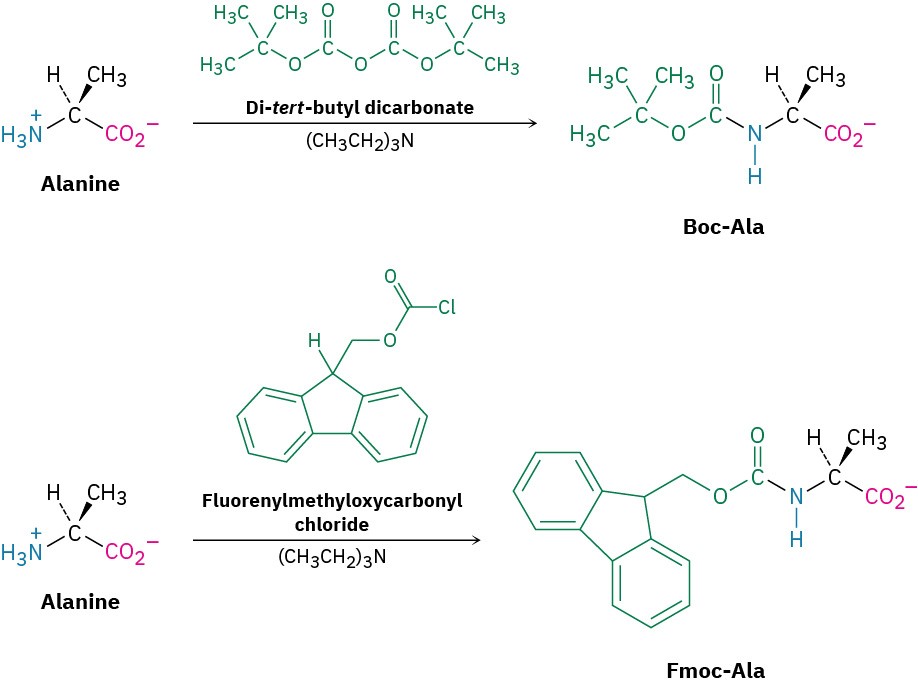
Amino groups are sometimes protected as their tert-butyloxycarbonyl amide (Boc) or, more commonly, as their fluorenylmethyloxycarbonyl amide (Fmoc). The Boc protecting group is introduced by reaction of the amino acid with di-tert-butyl dicarbonate in a nucleophilic acyl substitution reaction and is removed by brief treatment with a strong acid such as trifluoroacetic acid, CF3CO2H. The Fmoc protecting group is introduced by reaction with fluorenylmethyloxycarbonyl chloride and is removed by treatment with a 20% solution of the amine piperidine in dimethylformamide as solvent.
Thus, five steps are needed to synthesize a dipeptide such as Ala-Leu:
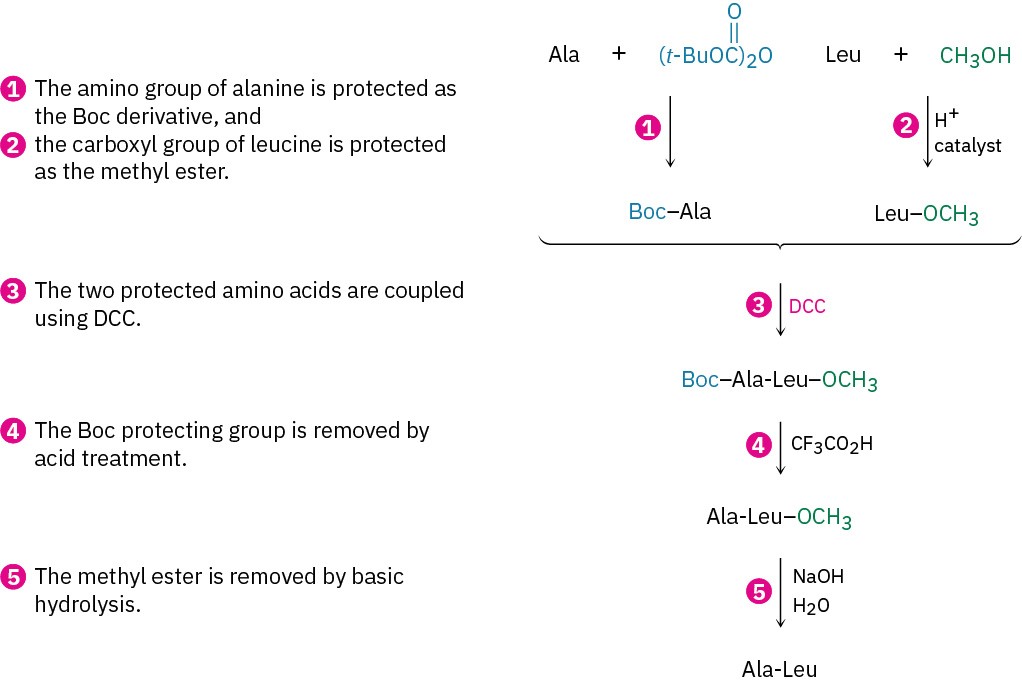
These steps can be repeated to add one amino acid at a time to the growing chain or to link two peptide chains together. Many remarkable achievements in peptide synthesis have been reported, including a complete synthesis of human insulin. Insulin is composed of two chains totaling 51 amino acids linked by two disulfide bridges. The three-dimensional structure of insulin, shown previously, was determined by Dorothy Crowfoot Hodgkin, a British chemist who received the 1964 Nobel Prize in Chemistry for her work on this and other complex biological molecules.
Problem 26-16
Show the mechanism for formation of a Boc derivative by reaction of an amino acid with di-
tert-butyl dicarbonate. Problem 26-17
Write all five steps required for the synthesis of Leu-Ala from alanine and leucine.

