18.3 Reactions of Ethers: Acidic Cleavage
Ethers are unreactive to many reagents used in organic chemistry, a property that accounts for their wide use as reaction solvents. Halogens, dilute acids, bases, and nucleophiles have no effect on most ethers. In fact, ethers undergo only one truly general reaction—they are cleaved by strong acids. Aqueous HBr and HI both work well, but HCl does not cleave ethers.
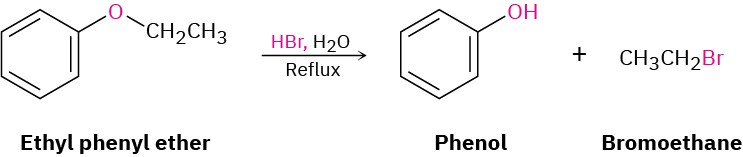
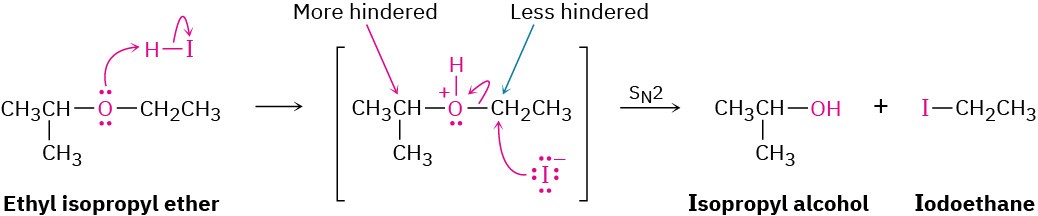
Acidic ether cleavages are typical nucleophilic substitution reactions and take place by either SN1 or SN2 mechanisms, depending on the structure of the substrate. Ethers with only primary and secondary alkyl groups react by an SN2 mechanism, in which I– or Br– reacts with the protonated ether at the less hindered site. This usually results in a selective cleavage into a single alcohol and a single alkyl halide. For example, ethyl isopropyl ether exclusively yields isopropyl alcohol and iodoethane on cleavage by HI because nucleophilic attack by iodide ion occurs at the less hindered primary site rather than at the more hindered secondary site.
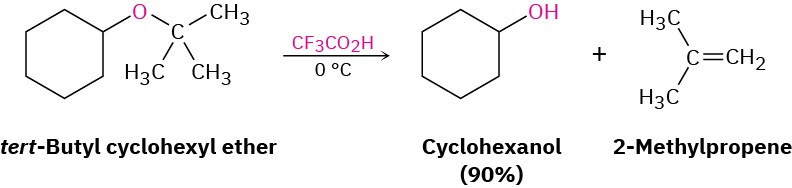
Ethers with a tertiary, benzylic, or allylic group cleave by either an SN1 or E1 mechanism because these substrates can produce stable intermediate carbocations. These reactions are often fast and take place at moderate temperatures. tert-Butyl ethers, for example, react by an E1 mechanism on treatment with trifluoroacetic acid at 0 °C. We’ll see in Section 26.7 that this reaction is often used in the laboratory synthesis of peptides.
 Worked Example 18.2Predicting the Product of an Ether Cleavage ReactionPredict the products of the following reaction:
Worked Example 18.2Predicting the Product of an Ether Cleavage ReactionPredict the products of the following reaction:
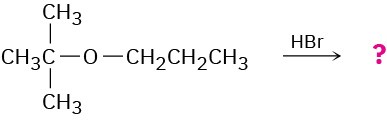
StrategyIdentify the substitution pattern of the two groups attached to oxygen—in this case a tertiary alkyl group and a primary alkyl group. Then recall the guidelines for ether cleavages. An ether with only primary and secondary alkyl groups usually undergoes cleavage by SN2 attack of a nucleophile on the less hindered alkyl group, but an ether with a tertiary alkyl group usually undergoes cleavage by an SN1 mechanism. In this case, an SN1 cleavage of the tertiary C–O bond will occur, giving 1-propanol and a tertiary alkyl bromide. In addition, a competitive E1 reaction leading to alkene might occur.Solution
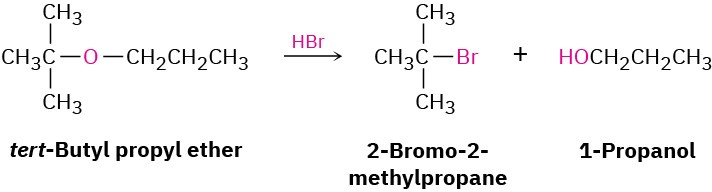
Problem 18-7
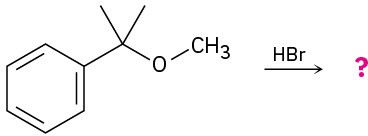
Predict the products of the following reactions:
(a)
(b)
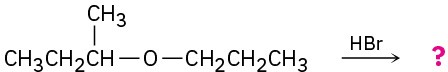
Problem 18-8
Write the mechanism of the acid-induced cleavage of tert-butyl cyclohexyl ether to yield cyclohexanol and 2-methylpropene.
Problem 18-9
Why are HI and HBr more effective than HCl in cleaving ethers? (See Section 11.3.)

