15.4 Aromatic Ions
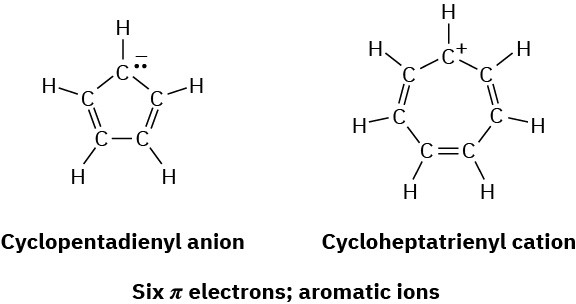
According to the Hückel criteria for aromaticity, a molecule must be cyclic, conjugated (nearly planar with a p orbital on each atom), and have 4n + 2 π electrons. Nothing in this definition says that the number of π electrons must be the same as the number of atoms in the ring or that the substance must be neutral. In fact, the numbers can differ and the substance can be an ion. Thus, both the cyclopentadienyl anion and the cycloheptatrienyl cation are aromatic even though both are ions and neither contains a six-membered ring.
To understand why the cyclopentadienyl anion and the cycloheptatrienyl cation are aromatic, imagine starting from the related neutral hydrocarbons, 1,3-cyclopentadiene and 1,3,5-cycloheptatriene, and removing one hydrogen from the saturated CH2 carbon in each. If that carbon then rehybridizes from sp3 to sp2, the resultant products would be fully conjugated, with a p orbital on every carbon. There are three ways in which the hydrogen might be removed.
- The hydrogen can be removed with both electrons (H:–) from the C–H bond, leaving a carbocation as product.
- The hydrogen can be removed with one electron (H·) from the C–H bond, leaving a carbon radical as product.
- The hydrogen can be removed with no electrons (H+) from the C–H bond, leaving a carbanion as product.
All the potential products formed by removing a hydrogen from 1,3-cyclopentadiene and from 1,3,5-cycloheptatriene can be drawn with numerous resonance structures, but Hückel’s rule predicts that only the six-π-electron cyclopentadienyl anion and cycloheptatrienyl cation can be aromatic. The other products are predicted by the 4n + 2 rule to be unstable and antiaromatic (Figure 15.7).
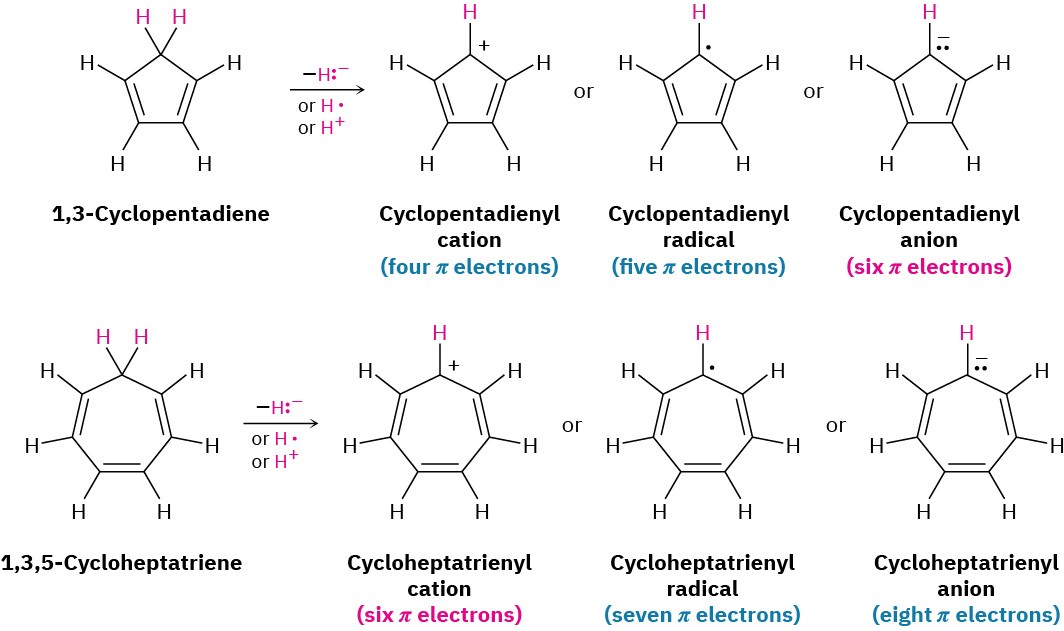
Figure 15.7 The aromatic six-π-electron cyclopentadienyl anion and the six-π– electron cycloheptatrienyl cation. The anion can be formed by removing a hydrogen ion (H+) from the CH2 group of 1,3-cyclopentadiene. The cation can be generated by removing a hydride ion (H:–) from the CH2 group of 1,3,5-cycloheptatriene.
In practice, both the four-π-electron cyclopentadienyl cation and the five-π-electron cyclopentadienyl radical are highly reactive and difficult to prepare. Neither shows any sign of the stability expected for an aromatic system. The six-π-electron cyclopentadienyl anion, by contrast, is easily prepared and remarkably stable (Figure 15.8a). In fact, the anion is so stable and easily formed that 1,3-cyclopentadiene is one of the most acidic hydrocarbons known, with pKa = 16, a value comparable to that of water!
In the same way, the seven-π-electron cycloheptatrienyl radical and eight-π-electron anion are reactive and difficult to prepare, while the six-π-electron cycloheptatrienyl cation is extraordinarily stable (Figure 15.8b). In fact, the cycloheptatrienyl cation was first prepared more than a century ago by reaction of 1,3,5-cycloheptatriene with Br2, although its structure was not recognized at the time.
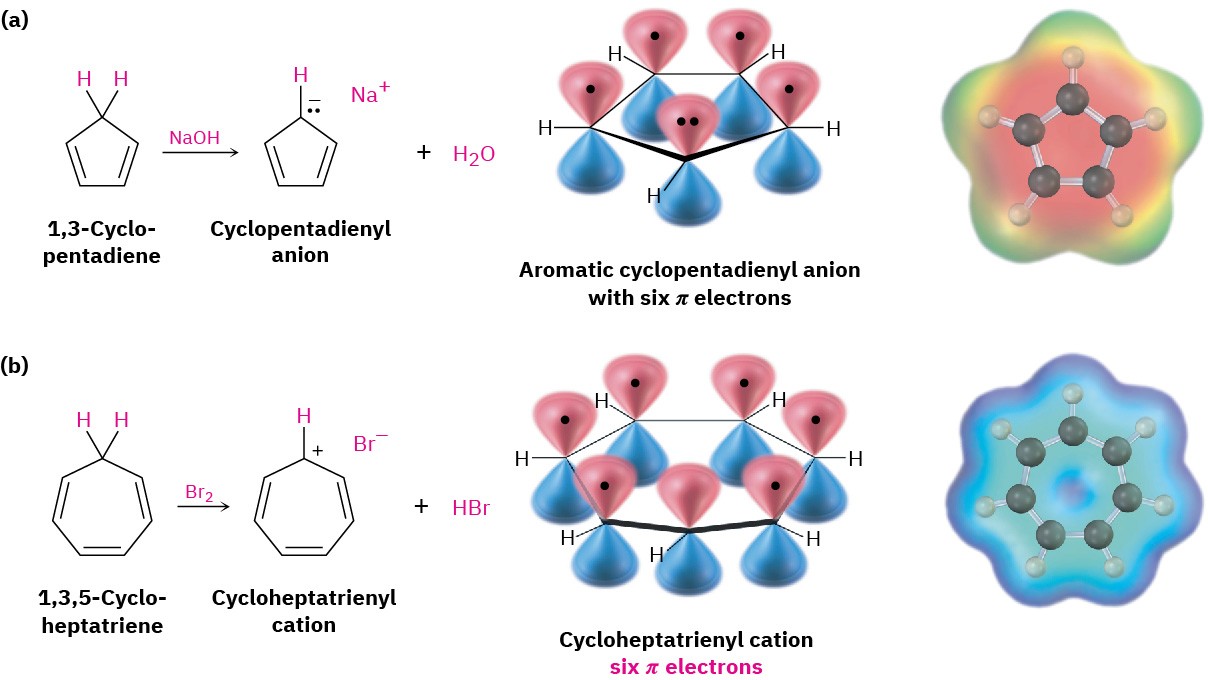
Figure 15.8 (a) The aromatic cyclopentadienyl anion, showing cyclic conjugation and six π electrons in five p orbitals, and (b) the aromatic cycloheptatrienyl cation, showing cyclic conjugation and six π electrons in seven p orbitals. Electrostatic potential maps indicate that both ions are symmetrical, with the charge equally shared among all atoms in each ring.
Problem 15-6
Draw the five resonance structures of the cyclopentadienyl anion. Are all carbon–carbon bonds equivalent? How many absorption lines would you expect to see in the 1H NMR and 13C NMR spectra of the anion?
Problem 15-7
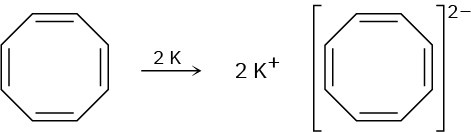
Cyclooctatetraene readily reacts with potassium metal to form the stable cyclooctatetraene dianion, C8H82–. Why do you suppose this reaction occurs so easily? What geometry do you expect for the cyclooctatetraene dianion?
Problem 15-8
The relative energy levels of the five π molecular orbitals of the cyclopentadienyl system are similar to those in benzene. That is, there is a single lowest-energy MO, above which the orbitals come in degenerate pairs. Draw a diagram like that in Figure 15.6, and tell which of the five orbitals are occupied in the cation, radical, and anion.

