18.8 Spectroscopy of Ethers
Infrared Spectroscopy
Ethers are difficult to identify by IR spectroscopy. Although they show an absorption due to C–O single-bond stretching in the range 1050 to 1150 cm–1, many other kinds of absorptions occur in the same range. Figure 18.3 shows the IR spectrum of diethyl ether and identifies the C–O stretch. Phenyl alkyl ethers show two strong absorbances for C–O stretching at 1050 and 1250 cm–1. Figure 18.4 shows the IR spectrum of anisole.
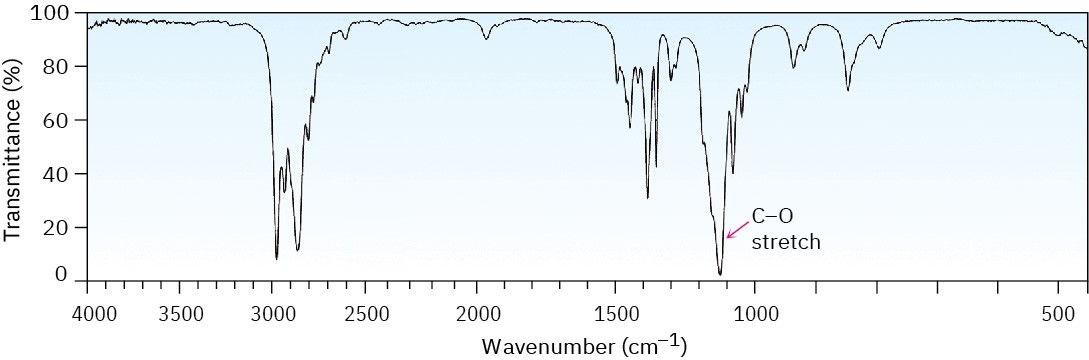
Figure 18.3 The infrared spectrum of diethyl ether, CH3CH2OCH2CH3.
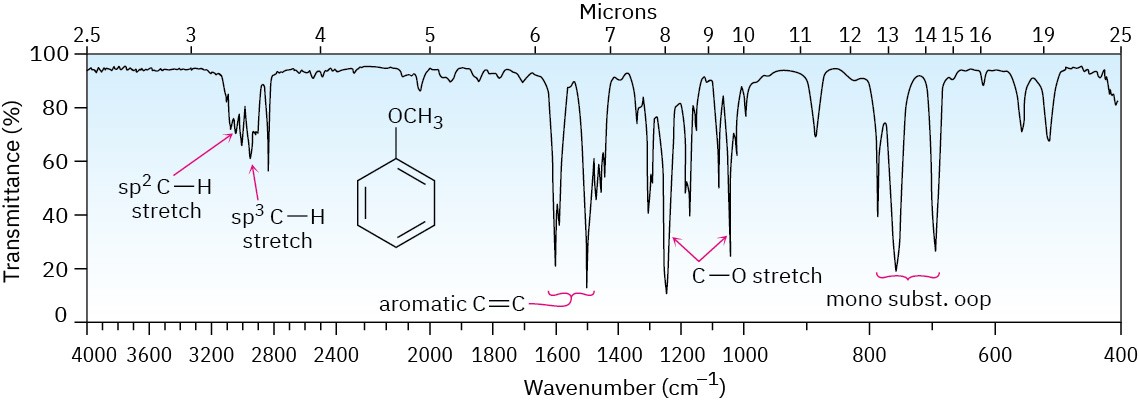
Figure 18.4 The infrared spectrum of anisole.
Nuclear Magnetic Resonance Spectroscopy
Hydrogens on carbon next to an ether oxygen are shifted downfield from the normal alkane resonance and show 1H NMR absorptions in the region 3.4 to 4.5 δ. This downfield shift is clearly seen in the spectrum of dipropyl ether shown in Figure 18.5.
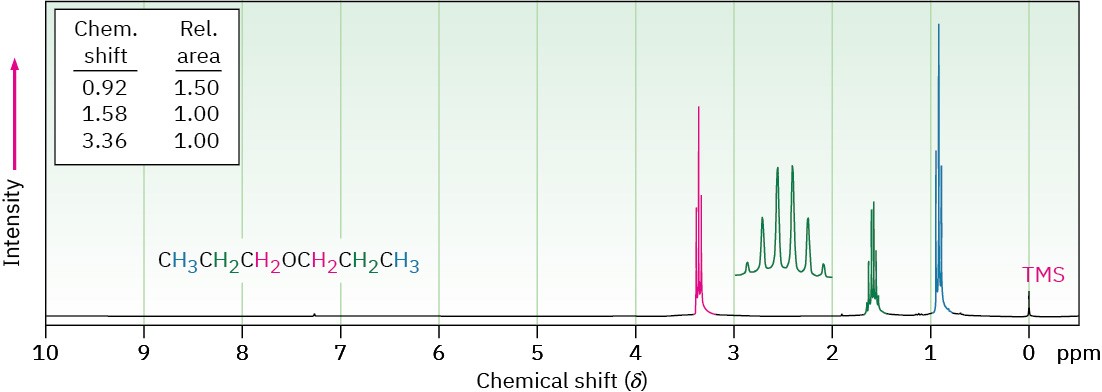
Figure 18.5 The 1H NMR spectrum of dipropyl ether. Protons on carbon next to oxygen are shifted downfield to 3.4 δ.
Epoxides absorb at a slightly higher field than other ethers and show characteristic resonances at 2.5 to 3.5 δ in their 1H NMR spectra, as indicated for 1,2-epoxypropane in Figure 18.6. The methylene protons of this epoxide are diastereotopic, and display complex splitting (see Section 13.7 and Section 13.8).
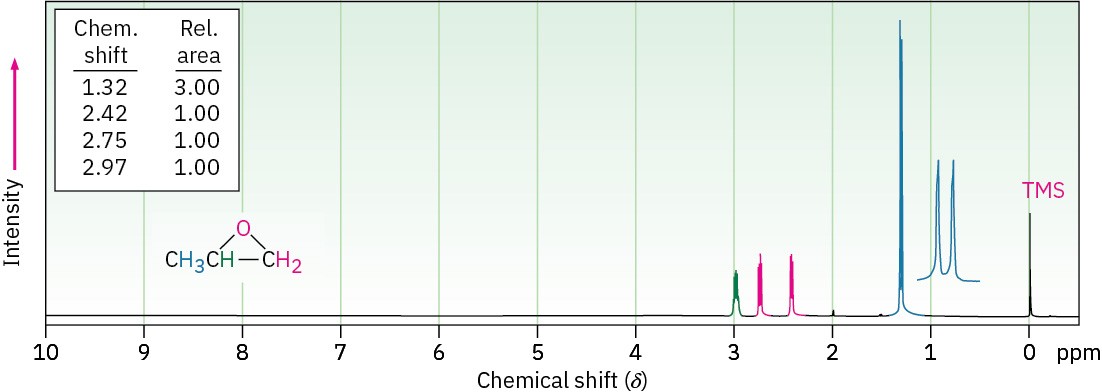
Figure 18.6 The 1H NMR spectrum of 1,2-epoxypropane.
Ether carbon atoms also exhibit a downfield shift in the 13C NMR spectrum, where they usually absorb in the 50 to 80 δ range. For example, the carbon atoms next to oxygen in methyl propyl ether absorb at 58.5 and 74.8 δ. Similarly, the methyl carbon in anisole absorbs at 54.8 δ.
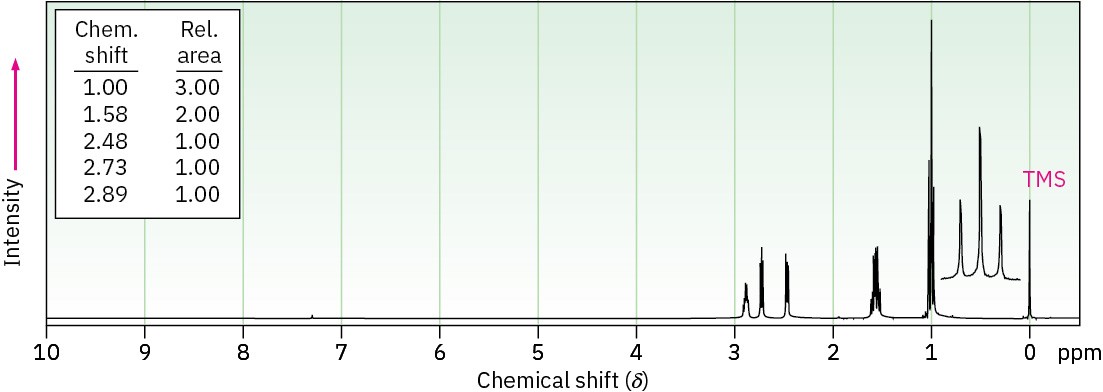
Problem 18-18
The 1H NMR spectrum shown is that of a cyclic ether with the formula C4H8O. Propose a structure.
Additional Problems 18 • Additional Problems 18 • Additional Problems Visualizing Chemistry Problem 18-19
Give IUPAC names for the following compounds (red = O; reddish brown = Br; yellow = S): (a)
(b)
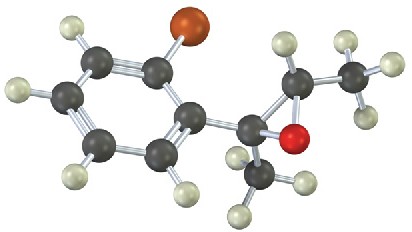
(c)
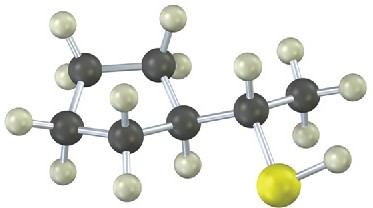
Problem 18-20
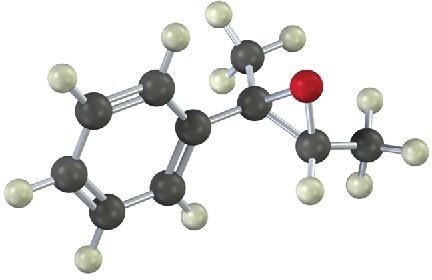
Show the product, including stereochemistry, that would result from reaction of the following epoxide with HBr:
Problem 18-21
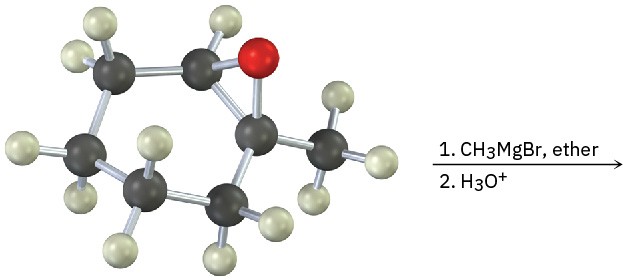
Show the product, including stereochemistry, of the following reaction:
Problem 18-22
Treatment of the following alkene with a peroxyacid yields an epoxide different from that obtained by reaction with aqueous Br2 followed by base treatment. Propose structures for the two epoxides, and explain the result.
Mechanism Problems
Problem 18-23
Predict the product(s) and provide the mechanism for each of the following reactions. (a)
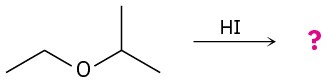
(b)
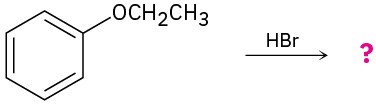
Problem 18-24
Predict the product(s) and provide the mechanism for each of the following reactions. (a)
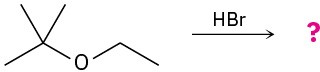
(b)
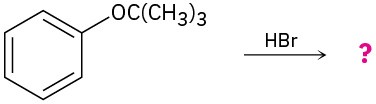
Problem 18-25
Predict the product(s) and provide the mechanism for each of the following two-step processes.
(a)
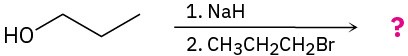
(b)
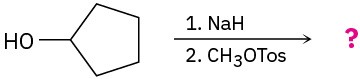
Problem 18-26
The alkoxymercuration of alkenes involves the formation of an organomercury intermediate (I), which is reduced with NaBH4 to give an ether product. Predict the ether product and provide the mechanism for the following reaction.
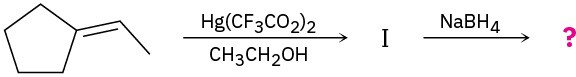
Problem 18-27
Predict the product(s) and provide the mechanism for the following reactions: (a)
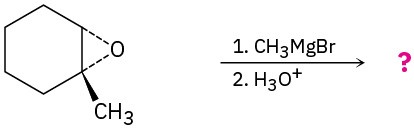
(b)
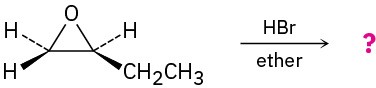
Problem 18-28
Predict the product(s) and provide the mechanism for each of the following reactions. (a)
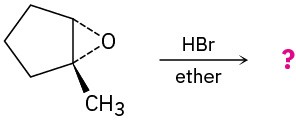
(b)
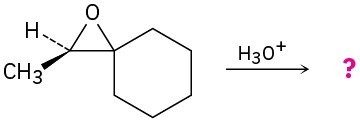
Problem 18-29
In the formation of the prepolymer used to make epoxy resins, a bisphenol reacts with epichlorohydrin in the presence of a base. Show the product and mechanism when two moles of phenol react with epichlorohydrin.
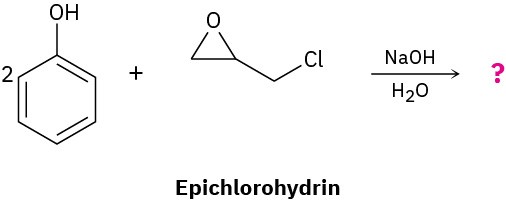
Problem 18-30
Ethers undergo an acid-catalyzed cleavage reaction when treated with the Lewis acid BBr3 at room temperature. Propose a mechanism for the reaction.
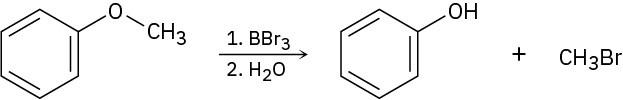
Problem 18-31
Treatment of 1,1-diphenyl-1,2-epoxyethane with aqueous acid yields diphenyl acetaldehyde as the major product. Propose a mechanism for the reaction.
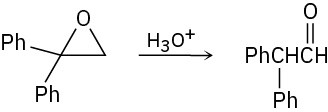
Problem 18-32
Fluoxetine, a heavily prescribed antidepressant marketed under the name Prozac, can be prepared by a route that begins with reaction between a phenol and an alkyl chloride.
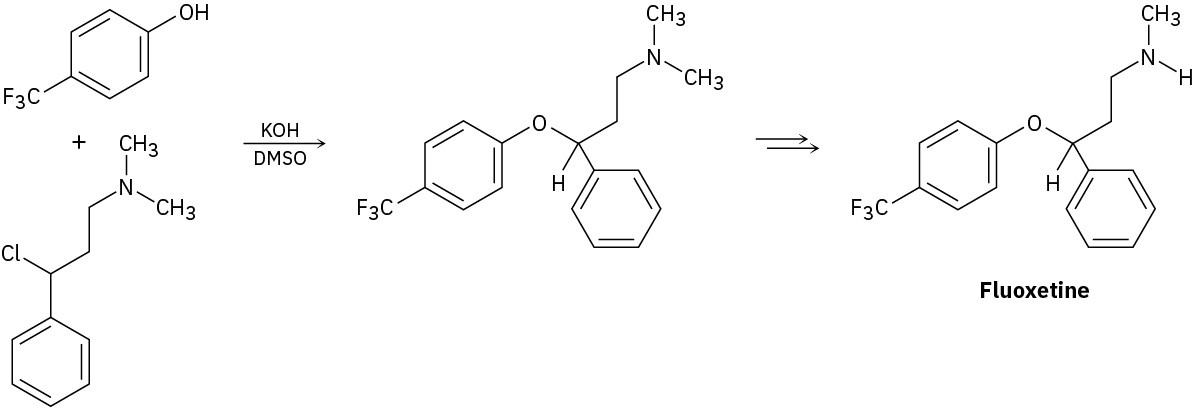
(a)
The rate of the reaction depends on both phenol and alkyl halide. Is this an SN1 or an SN2 reaction? Show the mechanism.
(b)
The physiologically active enantiomer of fluoxetine has (S) stereochemistry. Based on your answer in part (a), draw the structure of the alkyl chloride you would need, showing the correct stereochemistry.
Problem 18-33
When 2-methyl-2,5-pentanediol is treated with sulfuric acid, dehydration occurs and 2,2- dimethyltetrahydrofuran is formed. Suggest a mechanism for this reaction. Which of the two oxygen atoms is most likely to be eliminated, and why?

Problem 18-34
Methyl aryl ethers, such as anisole, are cleaved to iodomethane and a phenoxide ion by treatment with LiI in hot DMF. Propose a mechanism for this reaction.
Problem 18-35
The herbicide acifluorfen can be prepared by a route that begins with reaction between a phenol and an aryl fluoride. Propose a mechanism.
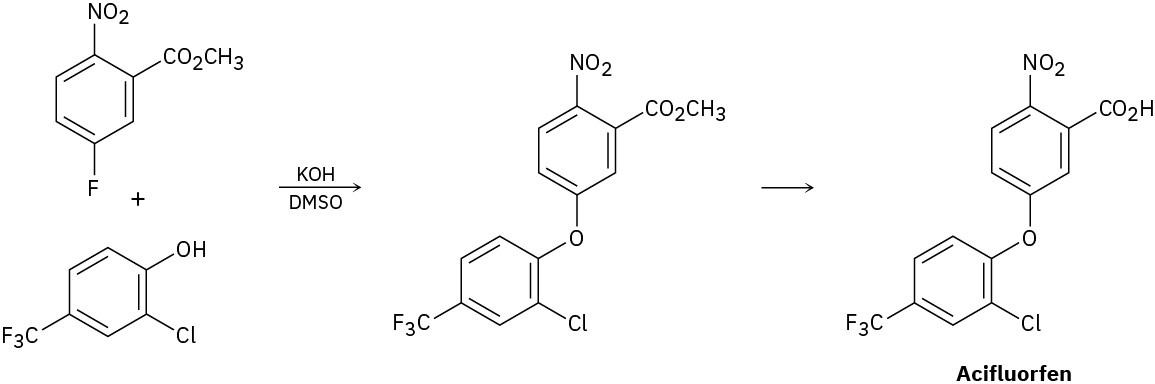
Problem 18-36
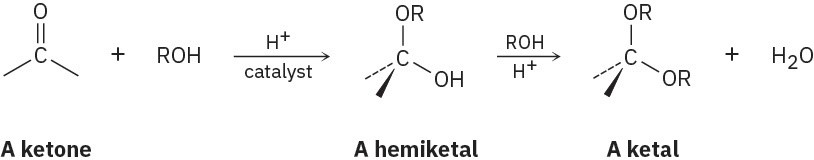
Aldehydes and ketones undergo acid-catalyzed reaction with alcohols to yield hemiacetals, from aldehydes or ketals with ketones compounds that have one alcohol-like oxygen and one ether-like oxygen bonded to the same carbon. Further reaction of a hemiacetal with alcohol then yields an acetal, a compound that has two ether-like oxygens bonded to the same carbon.
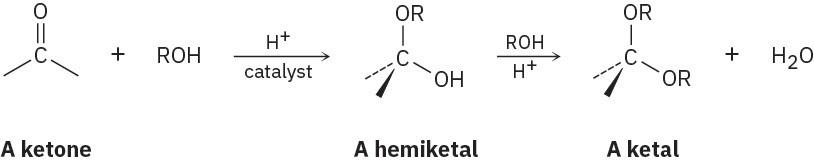
(a)
Show the structures of the hemiketal and ketal you would obtain by reaction of cyclohexanone with ethanol.
(b)
Propose a mechanism for the conversion of a hemiacetal into a ketal. Problem 18-37
Propose a mechanism to account for the following transformation. What two kinds of reactions are occurring?
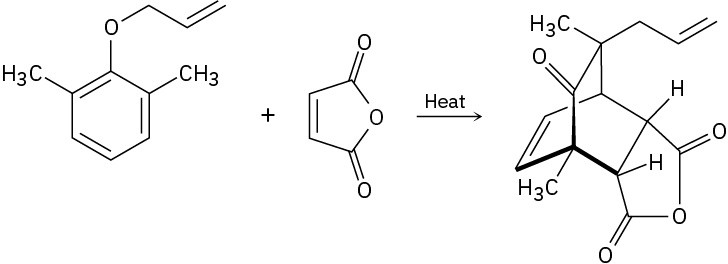
Naming Ethers
Problem 18-38
Draw structures corresponding to the following IUPAC names: (a)
Ethyl 1-ethylpropyl ether (b)
Di(p-chlorophenyl) ether (c)
3,4-Dimethoxybenzoic acid (d) Cyclopentyloxycyclohexane (e)
4-Allyl-2-methoxyphenol (eugenol; from oil of cloves) Problem 18-39
Give IUPAC names for the following structures:
(a)
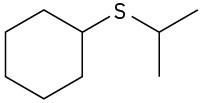
(b)
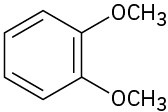
(c)
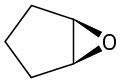
(d)
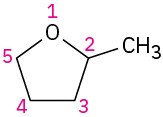
(e)
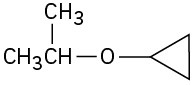
(f)
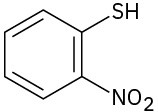
(g)
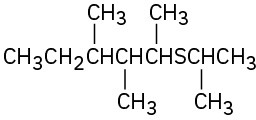
(h)
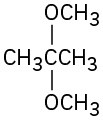
(i)
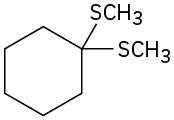
Synthesizing Ethers
Problem 18-40
How would you prepare the following ethers? (a)
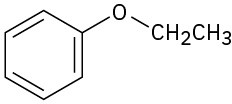
(b)
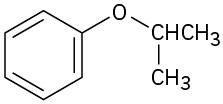
(c)
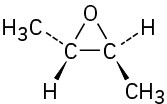
(d)
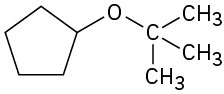
(e)
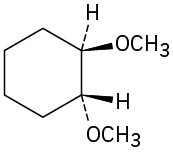
(f)
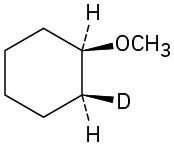
Problem 18-41
How would you prepare the following compounds from 1-phenylethanol? (a)
Methyl 1-phenylethyl ether (b)
Phenylepoxyethane (c)
tert-Butyl 1-phenylethyl ether (d)
1-Phenylethanethiol Problem 18-42
tert-Butyl ethers can be prepared by the reaction of an alcohol with 2-methylpropene in the presence of an acid catalyst. Propose a mechanism for this reaction.
Problem 18-43
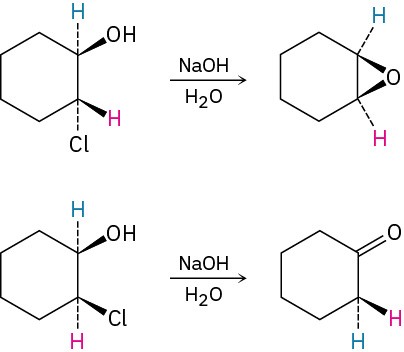
Treatment of trans-2-chlorocyclohexanol with NaOH yields 1,2-epoxycyclohexane, but reaction of the cis isomer under the same conditions yields cyclohexanone. Propose mechanisms for both reactions, and explain why the different results are obtained.
Reactions of Ethers and Epoxides
Problem 18-44
Predict the products of the following ether cleavage reactions: (a)
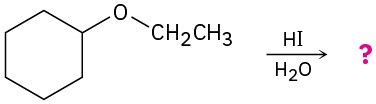
(b)
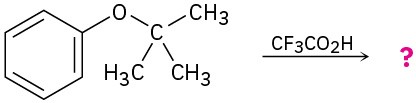
(c)
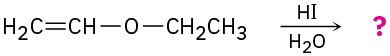
(d)
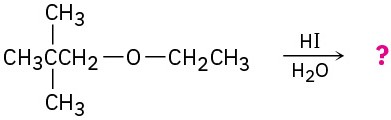
Problem 18-45
How would you carry out the following transformations? More than one step may be required.
(a)
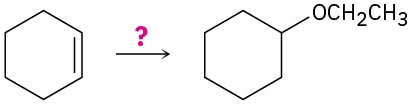
(b)
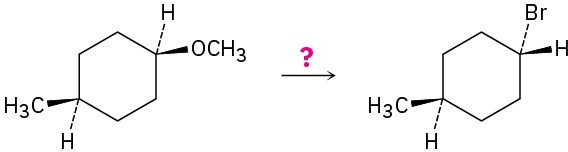
(c)
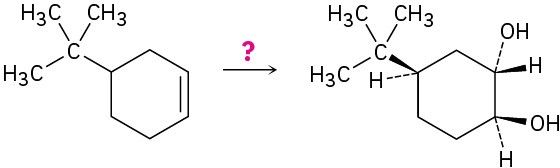
(d)

(e)

Problem 18-46
What product would you expect from cleavage of tetrahydrofuran with HI? Problem 18-47
Write the mechanism of the hydrolysis of cis-5,6-epoxydecane by reaction with aqueous acid. What is the stereochemistry of the product, assuming normal backside SN2 attack?
Problem 18-48
What is the stereochemistry of the product from acid-catalyzed hydrolysis of trans-5,6- epoxydecane? How does the product differ from that formed in Problem 18-47?
Problem 18-49
Acid-catalyzed hydrolysis of a 1,2-epoxycyclohexane produces a trans-diaxial 1,2-diol. What product would you expect to obtain from acidic hydrolysis of cis-3-tert-butyl-1,2- epoxycyclohexane? (Recall that the bulky tert-butyl group locks the cyclohexane ring into a specific conformation.)
Problem 18-50
Imagine that you have treated (2R,3R)-2,3-epoxy-3-methylpentane with aqueous acid to carry out a ring-opening reaction.

(a)
Draw the epoxide, showing stereochemistry. (b)
Draw and name the product, showing stereochemistry. (c)
Is the product chiral? Explain. (d)
Is the product optically active? Explain. Problem 18-51
Epoxides are reduced by treatment with lithium aluminum hydride to yield alcohols. Propose a mechanism for this reaction.
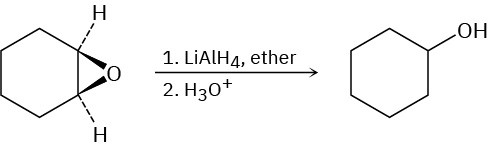
Problem 18-52
Show the structure and stereochemistry of the alcohol that would result if 1,2- epoxycyclohexane were reduced with lithium aluminum deuteride, LiAlD4 (Problem 18- 51).
Spectroscopy
Problem 18-53
The red fox (Vulpes vulpes) uses a chemical communication system based on scent marks in urine. One component of fox urine is a sulfide whose mass spectrum has M+ = 116. IR spectroscopy shows an intense band at 890 cm–1, and 1H NMR spectroscopy reveals the following peaks:
- 1.74 δ (3 H, singlet); 2.11 δ (3 H, singlet); 2.27 δ (2 H, triplet, J = 4.2 Hz); 2.57 δ (2 H, triplet, J = 4.2 Hz); 4.73 δ (2 H, broad)
Propose a structure consistent with these data. [Note: (CH3)2S absorbs at 2.1 δ]. Problem 18-54
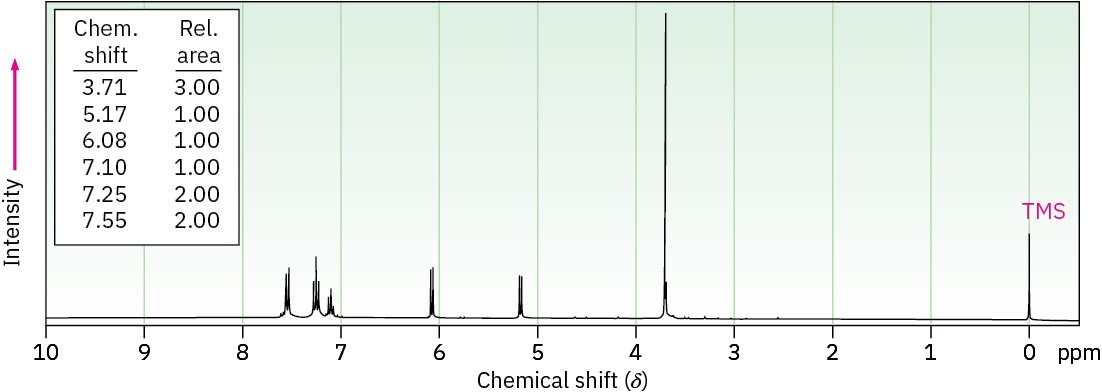
Anethole, C10H12O, a major constituent of the oil of anise, has the 1H NMR spectrum shown. On careful oxidation with Na2Cr2O7, anethole yields p-methoxybenzoic acid. What is the structure of anethole? Assign all peaks in the NMR spectrum, and account for the observed splitting patterns.
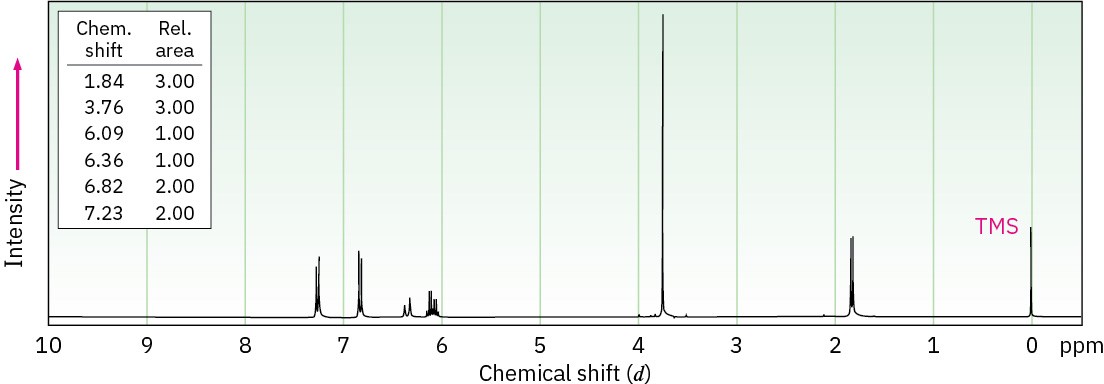
Problem 18-55
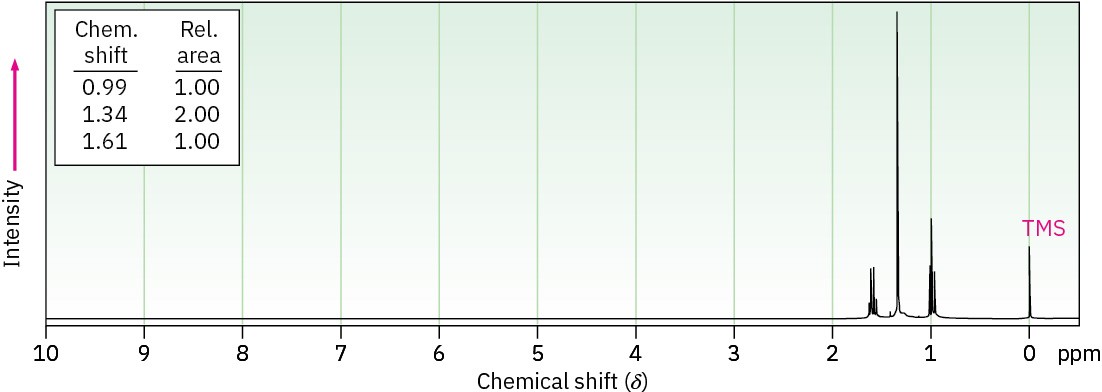
Propose structures for compounds that have the following 1H NMR spectra: (a)
C5H12S (An –SH proton absorbs near 1.6 δ.)
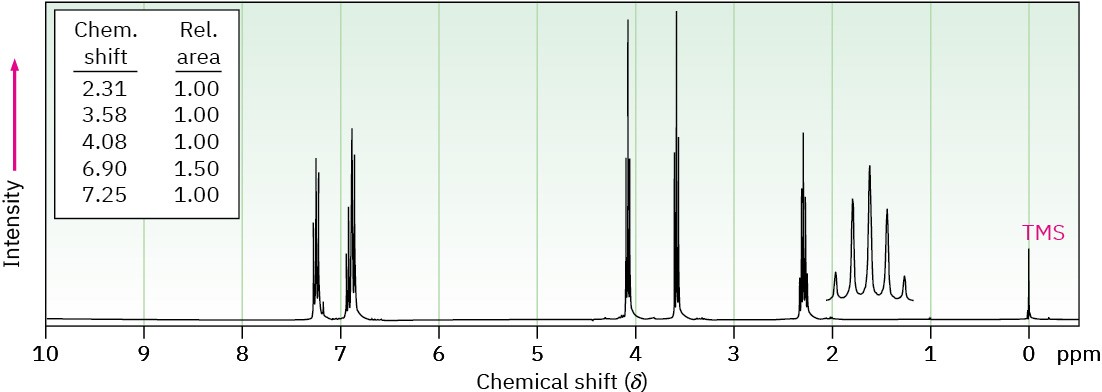
(b) C9H11BrO
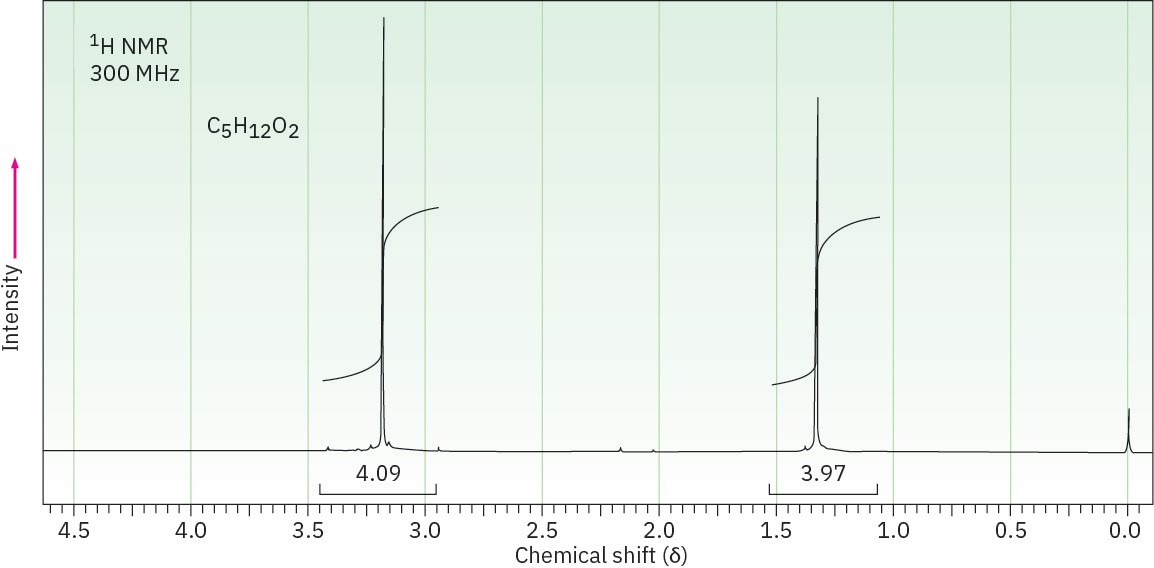
(c) C5H12O2
General Problems
Problem 18-56
Predict the products of the following reactions:
(a)
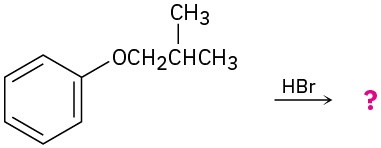
(b)
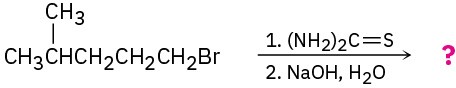
(c)
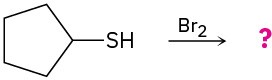
(d)
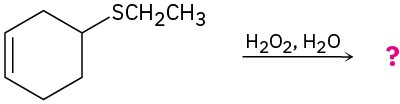
Problem 18-57
How would you synthesize anethole (Problem 18-54) from phenol? Problem 18-58
How could you prepare benzyl phenyl ether from benzene and phenol? More than one step is required.
Problem 18-59
Meerwein’s reagent, triethyloxonium tetrafluoroborate, is a powerful ethylating agent that converts alcohols into ethyl ethers at neutral pH. Show the reaction of Meerwein’s reagent with cyclohexanol, and account for the fact that trialkyloxonium salts are much more reactive alkylating agents than alkyl iodides.
(CH3CH2)3O+ BF4–Meerwein’s reagent
Problem 18-60
Safrole, a substance isolated from oil of sassafras, is used as a perfumery agent. Propose a synthesis of safrole from catechol (1,2-benzenediol).
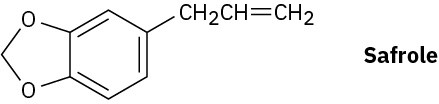
Problem 18-61
Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is much slower than the corresponding reaction with ethylene oxide. Suggest a reason for the difference in reactivity between oxetane and ethylene oxide.
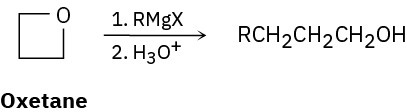
Problem 18-62
The Zeisel method is an old analytical procedure for determining the number of methoxyl groups in a compound. A weighed amount of the compound is heated with concentrated HI, ether cleavage occurs, and the iodomethane product is distilled off and passed into an alcohol solution of AgNO3, where it reacts to form a precipitate of silver iodide. The AgI is then collected and weighed, and the percentage of methoxyl groups in the sample is thereby determined. For example, 1.06 g of vanillin, the material responsible for the characteristic odor of vanilla, yields 1.60 g of AgI. If vanillin has a molecular weight of 152, how many methoxyl groups does it contain?
Problem 18-63
Disparlure, C19H38O, is a sex attractant released by the female spongy moth, Lymantria dispar. The 1H NMR spectrum of disparlure shows a large absorption in the alkane region, 1 to 2 δ, and a triplet at 2.8 δ. Treatment of disparlure, first with aqueous acid and then with KMnO4, yields two carboxylic acids identified as undecanoic acid and 6-methylheptanoic acid. (KMnO4 cleaves 1,2-diols to yield carboxylic acids.) Neglecting stereochemistry, propose a structure for disparlure. The actual compound is a chiral molecule with 7R,8S stereochemistry. Draw disparlure, showing the correct stereochemistry.
Problem 18-64
How would you synthesize racemic disparlure (Problem 18-63) from compounds having ten or fewer carbons?
Problem 18-65
How would you prepare o-hydroxyphenylacetaldehyde from phenol? More than one step is required.

Problem 18-66
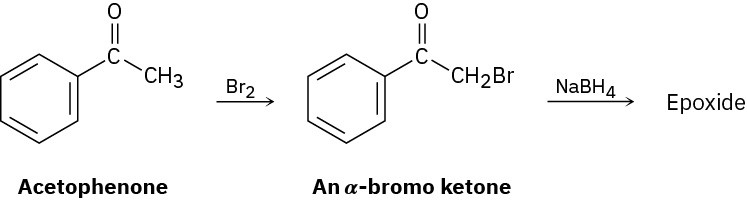
Identify the reagents a–e in the following scheme:
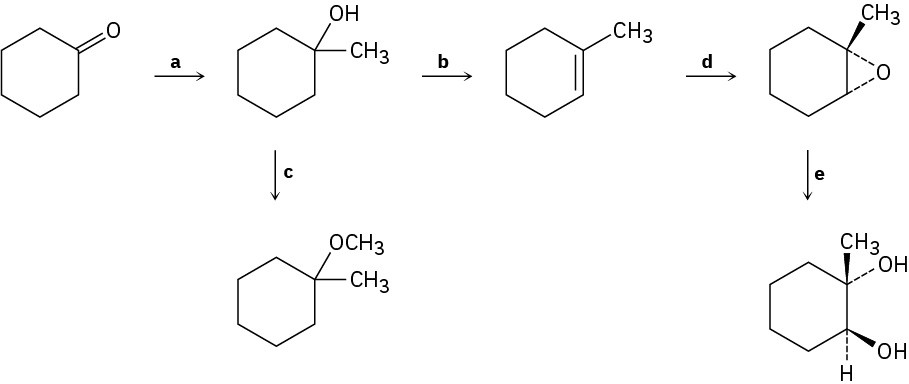
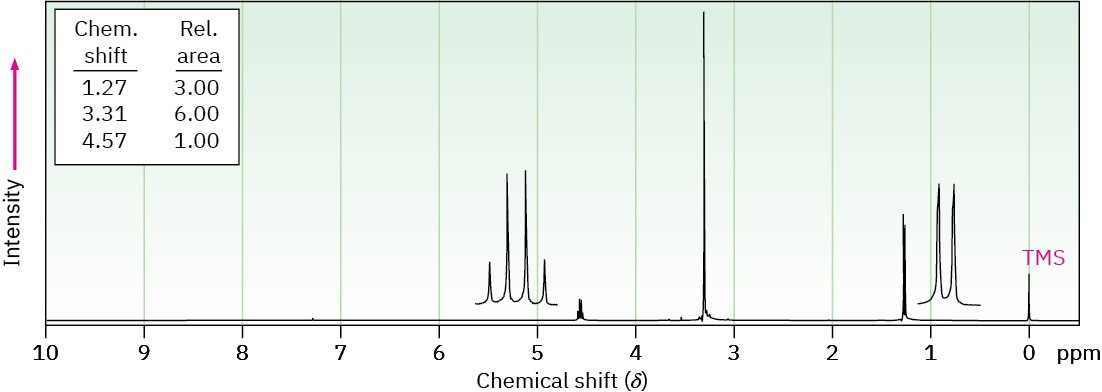
Problem 18-67
Propose structures for compounds that have the following 1H NMR spectra: (a)
C4H10O2
(b) C9H10O
Problem 18-68
We saw in Section 17.4 that ketones react with NaBH4 to yield alcohols. We’ll also see in Section 22.3 that ketones react with Br2 to yield α-bromo ketones. Perhaps surprisingly, treatment with NaBH4 of the α-bromo ketone from acetophenone yields an epoxide rather than a bromo alcohol. Show the structure of the epoxide, and explain its formation.