31.5 Olefin Metathesis Polymerization
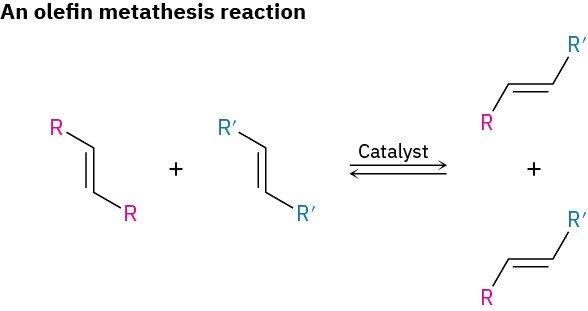
Perhaps the most important advance in polymer synthesis in recent years has been the development of olefin metathesis polymerization by Yves Chauvin of the Institut Français du Pétrole, Robert H. Grubbs of CalTech, and Richard R.Schrock of MIT who shared the 2005 Nobel Prize in Chemistry for their work. At its simplest, an olefin metathesis reaction is two olefins (alkenes) exchanging substituents on their double bonds.
Olefin metathesis catalysts, such as the Grubbs catalyst now in common use, contain a carbon–metal double bond (usually to ruthenium, Ru) and have the general structure M═CH─R. They function by reacting reversibly with an alkene to form a four-membered, metal-containing intermediate called a metallacycle, which immediately opens to give a different catalyst and a different alkene. The mechanism is shown in Figure 31.3.
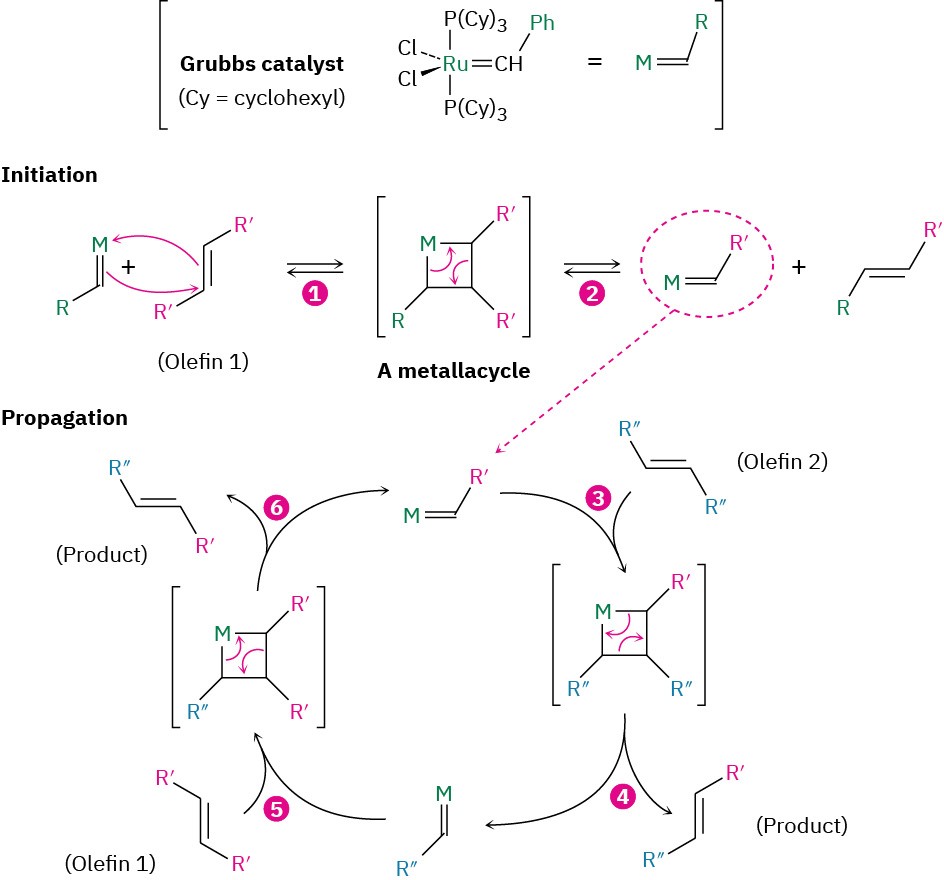
Figure 31.3 Mechanism of the olefin metathesis reaction. The process is initiated by a two-step sequence that involves (1) reaction of the catalyst and olefin 1 to give a four- membered metallacycle intermediate, followed by (2) ring-opening to give a different form of catalyst that contains part of olefin 1. (3) Reaction of this new catalyst with olefin 2 gives another metallacycle intermediate, (4) which opens to give metathesis product and another form of catalyst. (5, 6) The repeating ring-forming and ring-opening steps then continue.
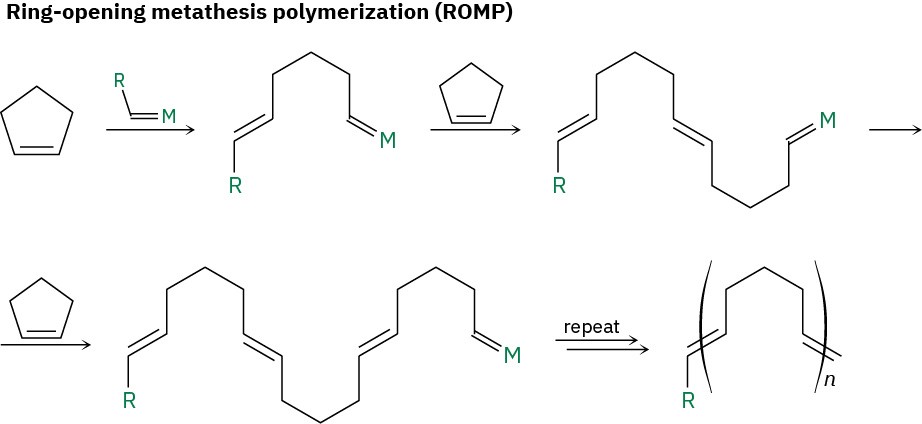
There are several methods for implementing the olefin metathesis reaction to prepare polymers. One method, called ring-opening metathesis polymerization, or ROMP, involves the use of a moderately strained cycloalkene, such as cyclopentene. The strain of the ring favors ring-opening, thereby driving formation of the open-chain product. The polymer that results has double bonds spaced regularly along the chain, allowing for either hydrogenation or further functionalization if desired.
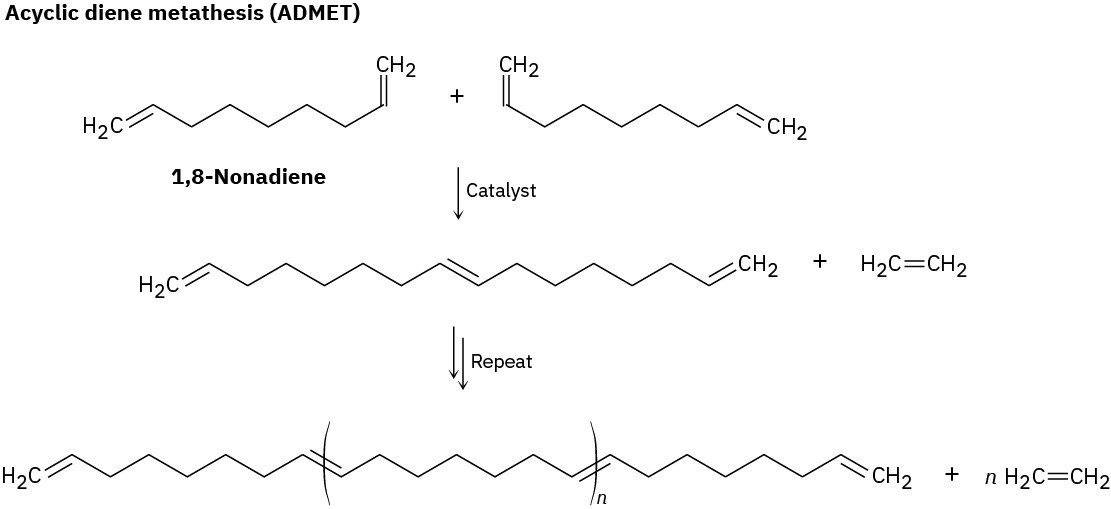
A second method of using olefin metathesis to prepare polymers is by acyclic diene metathesis, or ADMET. As the name suggests, ADMET involves olefin metathesis of an open-chain substrate with two double bonds at the ends of a long chain, such as 1,8- nonadiene. As the reaction proceeds, the gaseous ethylene by-product escapes, thereby driving the equilibrium toward polymer product. So efficient is this reaction that polymers with molecular weights as high as 80,000 amu have been prepared.
The ROMP and ADMET procedures are particularly valuable because the metathesis reaction is compatible with the presence in the olefin monomer of many different functional groups. In addition, the double bonds in the polymers provide still more flexibility for further manipulations. Among the commercial polymers produced by olefin metathesis are Vestenamer, used in the manufacture of tires and other molded rubber objects, and Norsorex, used in the automobile industry as a sealing material.
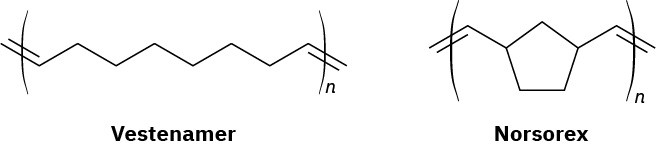
Problem 31-10
Look at the structures of Vestenamer and Norsorex and show how they might be made by olefin metathesis polymerization.

