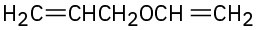18.1 Names and Properties of Ethers
Simple ethers with no other functional groups are named by identifying the two organic substituents and adding the word ether.
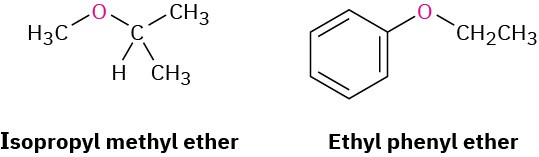
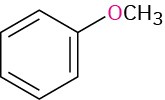
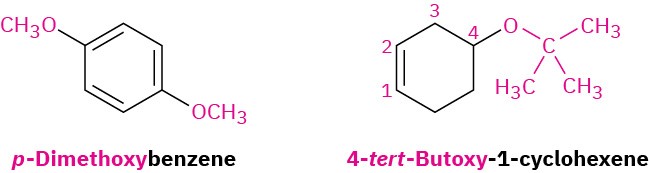
If other functional groups are present, the ether part is considered an alkoxy substituent. For example:
Like alcohols, ethers have nearly the same geometry as water. The R–O–R bonds have an approximately tetrahedral bond angle (112° in dimethyl ether), and the oxygen atom is sp3- hybridized.
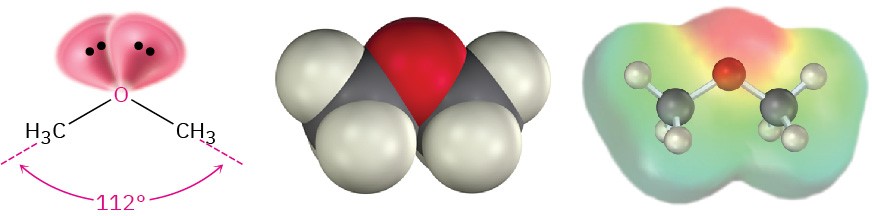
The electronegative oxygen atom gives ethers a slight dipole moment, and the boiling points of ethers are often slightly higher than the boiling points of comparable alkanes. Table 18.1 compares the boiling points of some common ethers and their corresponding hydrocarbons.
Table 18.1 Comparison of Boiling Points of Ethers and Hydrocarbons
|
Boiling point °C |
Hydrocarbon |
Boiling point °C |
|
|
CH3OCH3 |
–25 |
CH3CH2CH3 |
–45 |
|
CH3CH2OCH2CH3 |
34.6 |
CH3CH2CH2CH2CH3 |
36 |
|
|
65 |
|
49 |
|
|
158 |
|
136 |
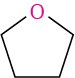
Ethers are relatively stable and unreactive in many respects, but some ethers react slowly with the oxygen in air to give peroxides, compounds that contain an O–O bond. The peroxides from low-molecular-weight ethers such as diisopropyl ether and tetrahydrofuran are explosive and extremely dangerous, even in small amounts. Ethers are very useful as solvents in the laboratory, but they must always be used cautiously and should not be stored for long periods of time.
Problem 18-1
Name the following ethers: (a)
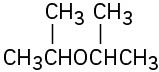
(b)
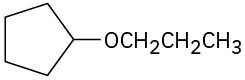
(c)
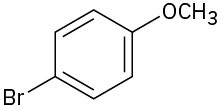
(d)
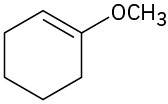
(e)
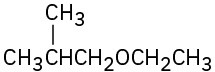
(f)