6.8 Describing a Reaction: Bond Dissociation Energies
We’ve just seen that heat is released (negative ΔH) when a bond is formed because the products are more stable and have stronger bonds than the reactants. Conversely, heat is absorbed (positive ΔH) when a bond is broken because the products are less stable and have weaker bonds than the reactants. The amount of energy needed to break a given bond to produce two radical fragments when the molecule is in the gas phase at 25 °C is a quantity called the bond strength, or bond dissociation energy (D).
Each specific bond has its own characteristic strength, and extensive tables of such data are available. For example, a C−H bond in methane has a bond dissociation energy D = 439.3 kJ/mol (105.0 kcal/mol), meaning that 439.3 kJ/mol must be added to break a C−H bond of methane to give the two radical fragments ·CH3 and ·H. Conversely, 439.3 kJ/mol of energy is released when a methyl radical and a hydrogen atom combine to form methane. Table
6.3 lists some other bond strengths.
Table 6.3 Some Bond Dissociation Energies, D
|
Bond |
D (kJ/mol) |
Bond |
D (kJ/mol) |
Bond |
D (kJ/mol) |
|
H―H |
436 |
(CH3)2CH―H |
410 |
C2H5―CH3 |
370 |
|
H―F |
570 |
(CH3)2CH―CI |
354 |
(CH3)2CH―CH3 |
369 |
|
H―CI |
431 |
(CH3)2CH―Br |
299 |
(CH3)3C―CH3 |
363 |
|
H―Br |
366 |
(CH3)3C―H |
400 |
H2C═CH―CH3 |
426 |
|
H―I |
298 |
(CH3)3C―CI |
352 |
H2C═CHCH2―CH3 |
318 |
|
CI―CI |
242 |
(CH3)3C―Br |
293 |
H2C═CH2 |
728 |
|
Br―Br |
194 |
(CH3)3C―I |
227 |
|
427 |
|
I―I |
152 |
H2C═CH―H |
464 |
|
325 |
|
CH3―H |
439 |
H2C═CH―CI |
396 |
|
374 |
|
CH3―CI |
350 |
H2C═CHCH2―H |
369 |
HO―H |
497 |
|
CH3―Br |
294 |
H2C═CHCH2―CI |
298 |
HO―OH |
211 |
|
CH3―I |
239 |
|
472 |
CH3O―H |
440 |
|
D (kJ/mol) |
Bond |
D (kJ/mol) |
Bond |
D (kJ/mol) |
|
|
CH3―OH |
385 |
|
400 |
CH3S―H |
366 |
|
CH3―NH2 |
386 |
|
375 |
C2H5O―H |
441 |
|
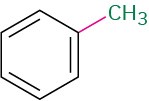
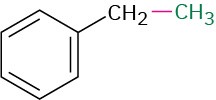
C2H5―H |
421 |
|
300 |
|
352 |
|
C2H5―CI |
352 |
|
336 |
CH3CH2O―CH3 |
355 |
|
C2H5―Br |
293 |
|
464 |
NH2―H |
450 |
|
C2H5―I |
233 |
HC≡C―H |
558 |
H―CN |
528 |
|
C2H5―OH |
391 |
CH3―CH3 |
377 |
|
|
Think again about the connection between bond strengths and chemical reactivity. In an exothermic reaction, more heat is released than is absorbed. But because making bonds in the products releases heat and breaking bonds in the reactants absorbs heat, the bonds in the products must be stronger than the bonds in the reactants. In other words, exothermic reactions are favored by products with strong bonds and by reactants with weak, easily broken bonds.
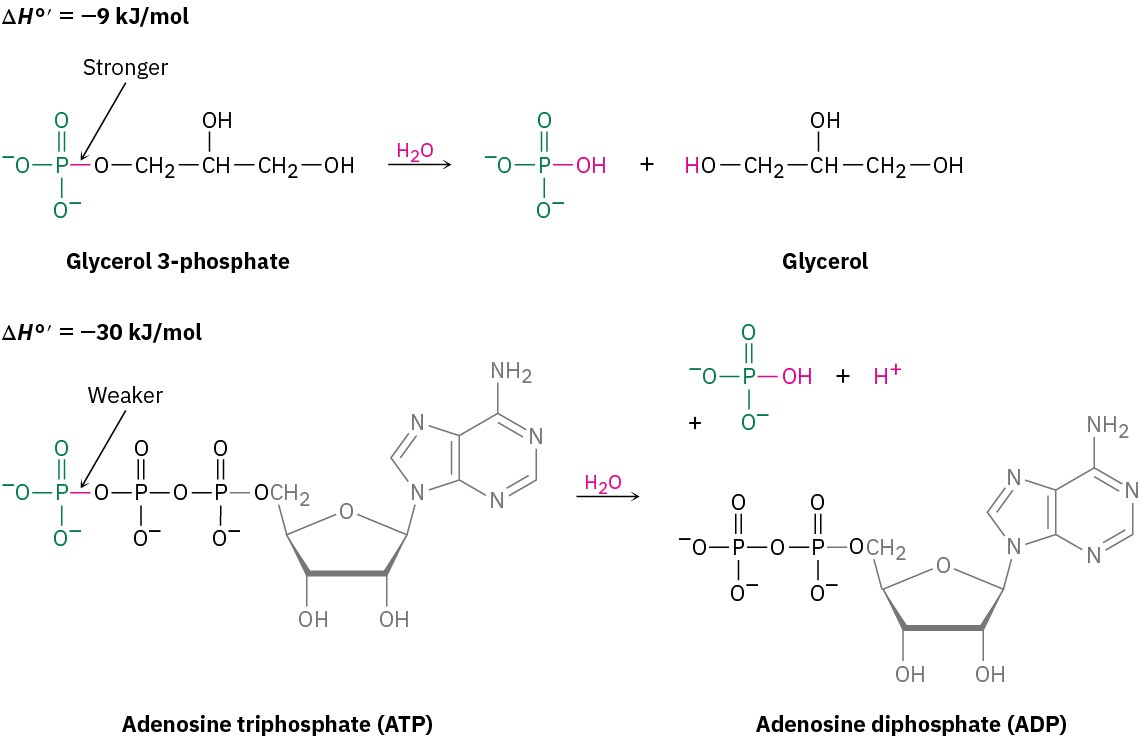
Sometimes, particularly in biochemistry, reactive substances that undergo highly exothermic reactions, such as ATP (adenosine triphosphate), are referred to as “energy- rich” or “high-energy” compounds. Such a label doesn’t mean that ATP is special or different from other compounds, it only means that ATP has relatively weak bonds that require a relatively small amount of heat to break, thus leading to a larger release of heat when a strong new bond forms in a reaction. When a typical organic phosphate such as glycerol 3-phosphate reacts with water, for instance, only 9 kJ/mol of heat is released (ΔH°′
= −9 kJ/mol), but when ATP reacts with water, 30 kJ/mol of heat is released (ΔH°′ = −30 kJ/mol). The difference between the two reactions is due to the fact that the bond broken in ATP is substantially weaker than the bond broken in glycerol 3-phosphate. We’ll see the metabolic importance of this reaction in later chapters.