14.4 The Diels–Alder Cycloaddition Reaction
Perhaps the most striking difference between conjugated and nonconjugated dienes is that conjugated dienes undergo an addition reaction with alkenes to yield substituted cyclohexene products. For example, 1,3-butadiene and 3-buten-2-one give 3-cyclohexenyl methyl ketone.
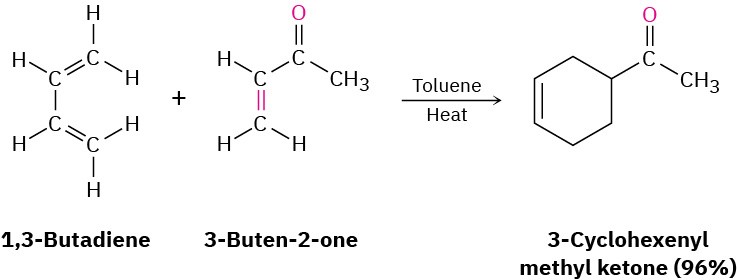
This process, named the Diels–Alder cycloaddition reaction after its discoverers, is extremely useful in the laboratory because it forms two carbon–carbon bonds in a single step and is one of the few general methods available for making cyclic molecules. (As the name implies, a cycloaddition reaction is one in which two reactants add together to give a cyclic product.) The 1950 Nobel Prize in Chemistry was awarded to Otto Diels and Kurt Alder in recognition of the importance of their discovery.
The mechanism of Diels–Alder cycloaddition is different from that of other reactions we’ve studied because it is neither polar nor radical. Rather, the Diels–Alder reaction is a pericyclic process. Pericyclic reactions, which we’ll discuss in more detail in Chapter 30, take place in a single step by a cyclic redistribution of bonding electrons. The two reactants simply join together through a cyclic transition state in which the two new C–C bonds form at the same time.
We can picture a Diels–Alder addition as occurring by head-on (σ) overlap of the two alkene p orbitals with the two p orbitals on carbons 1 and 4 of the diene (Figure 14.8). This is, of course, a cyclic orientation of the reactants.
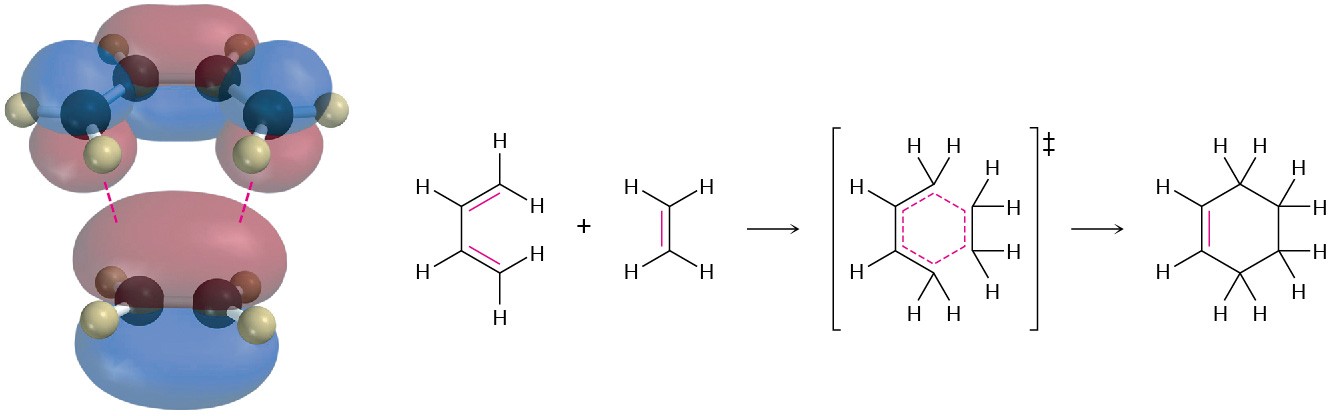
Figure 14.8 Mechanism of the Diels–Alder cycloaddition reaction. The reaction occurs in a single step through a cyclic transition state in which the two new C−C bonds form simultaneously.
In the Diels–Alder transition state, the two alkene carbons and carbons 1 and 4 of the diene rehybridize from sp2 to sp3 to form two new single bonds, while carbons 2 and 3 of the diene remain sp2-hybridized to form the new double bond in the cyclohexene product.
We’ll study this mechanism in more detail in Section 30.5 but will concentrate for the present on learning about the characteristics and uses of the Diels–Alder reaction.

