29.4 Biosynthesis of Fatty Acids
One of the most striking features of the common fatty acids is that they have an even number of carbon atoms (Table 27.1). This occurs because all fatty acids are derived biosynthetically from acetyl CoA by sequential addition of two-carbon units to a growing chain. The acetyl CoA, in turn, arises primarily from the metabolic breakdown of carbohydrates in the glycolysis pathway, which we’ll see in Section 29.5. Thus, dietary carbohydrates consumed in excess of immediate energy needs are turned into fats for storage.
As a general rule in biological chemistry, the anabolic pathway by which a substance is made is not the reverse of the catabolic pathway by which the same substance is degraded. The two paths must differ in some respects for both to be energetically favorable. Thus, the β-oxidation pathway for converting fatty acids into acetyl CoA and the biosynthesis of fatty acids from acetyl CoA are related but are not exact opposites. Differences include the identity of the acyl-group carrier, the stereochemistry of the β-hydroxyacyl reaction intermediate, and the identity of the redox coenzyme. FAD is used to introduce a double bond in β-oxidation, while NADPH is used to reduce the double bond in fatty-acid biosynthesis.
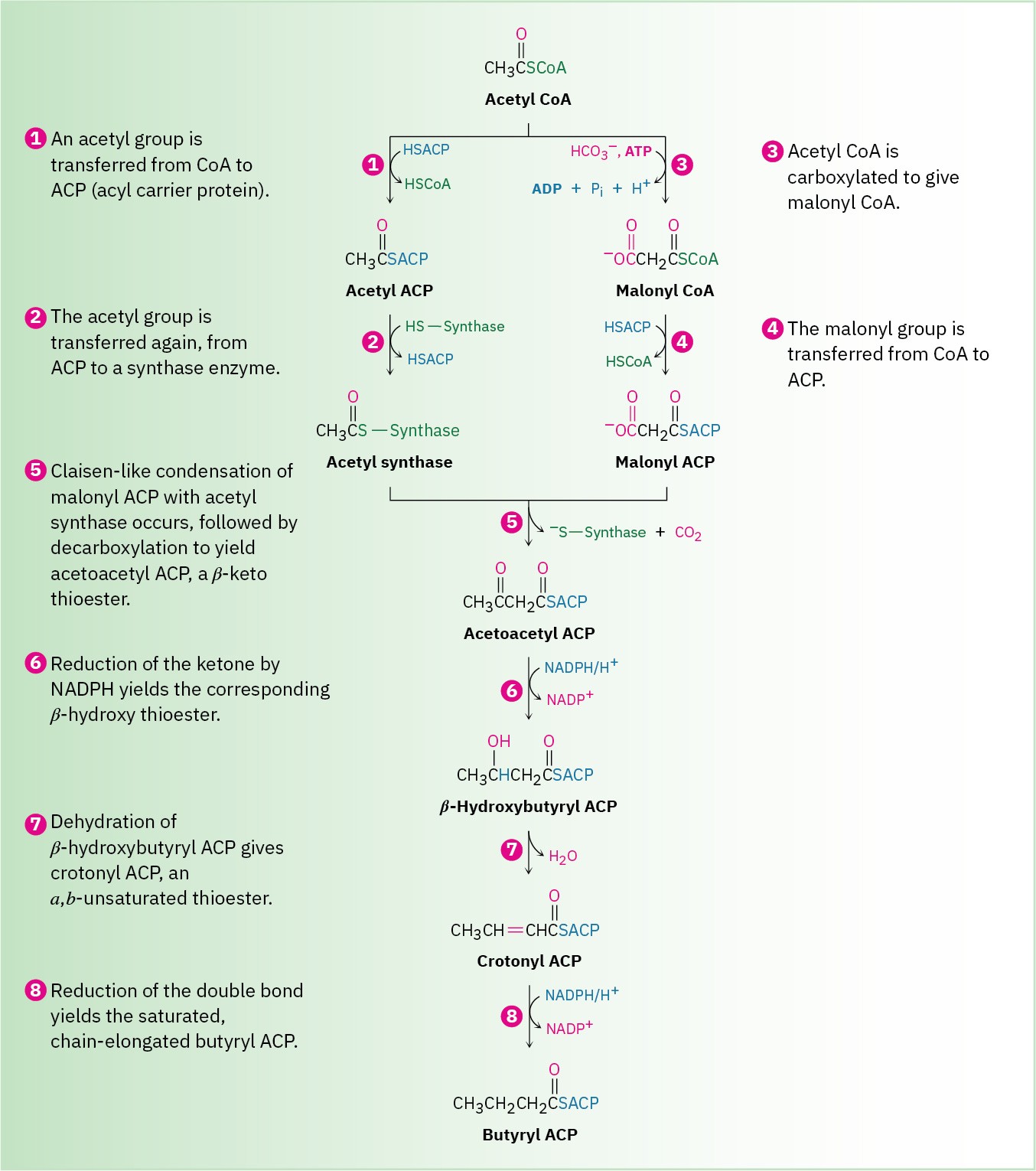
In bacteria, each step in fatty-acid synthesis is catalyzed by a separate enzyme. In vertebrates, however, fatty-acid synthesis is catalyzed by an immense, multienzyme complex called a synthase that contains two identical subunits of 2505 amino acids each and catalyzes all steps in the pathway. In fact, for an 18-carbon fatty acid, the synthase catalyzes 42 separate steps! An overview of fatty-acid biosynthesis is shown in Figure 29.6.
Steps 1–2 of Figure 29.6: Acyl Transfers
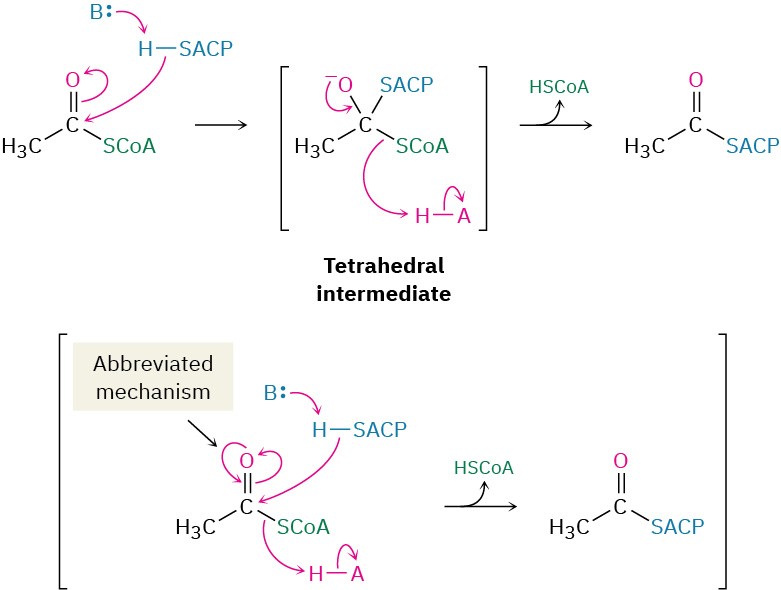
The starting material for fatty-acid biosynthesis is the thioester acetyl CoA, the final product of carbohydrate breakdown, as we’ll see in Section 29.6. The pathway begins with several priming reactions, which transport acetyl CoA and convert it into more reactive species. The first priming reaction is a nucleophilic acyl substitution reaction that converts acetyl CoA into acetyl ACP (acyl carrier protein).
Notice that the mechanism of the nucleophilic acyl substitution in step 1 can be given in an abbreviated form that saves space by not explicitly showing the tetrahedral reaction intermediate. Instead, electron movement is shown as a heart-shaped path around the carbonyl oxygen to imply the two steps of the full mechanism. Biochemists commonly use this kind of abbreviated format, and we’ll also use it on occasion through the rest of this chapter.
Figure 29.6 MECHANISM
The pathway for fatty-acid biosynthesis from the two-carbon precursor, acetyl CoA.
Individual steps are explained in the text.
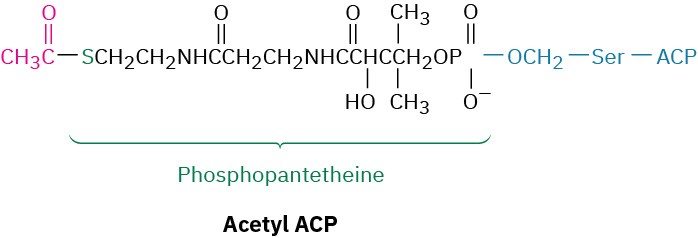
In bacteria, ACP is a small protein of 77 residues that transports an acyl group from one enzyme to another. In vertebrates, however, ACP appears to be a long arm on a multienzyme synthase complex, whose apparent function is to shepherd an acyl group from site to site within the complex. As in acetyl CoA, the acyl group in acetyl ACP is linked by a thioester bond to the sulfur atom of phosphopantetheine. The phosphopantetheine is in turn linked to ACP through the side-chain –OH group of a serine residue in the enzyme.
Step 2, another priming reaction, involves a further exchange of thioester linkages by another nucleophilic acyl substitution and results in covalent bonding of the acetyl group to a cysteine residue in the synthase complex that catalyzes the upcoming condensation step.
Steps 3–4 of Figure 29.6: Carboxylation and Acyl Transfer
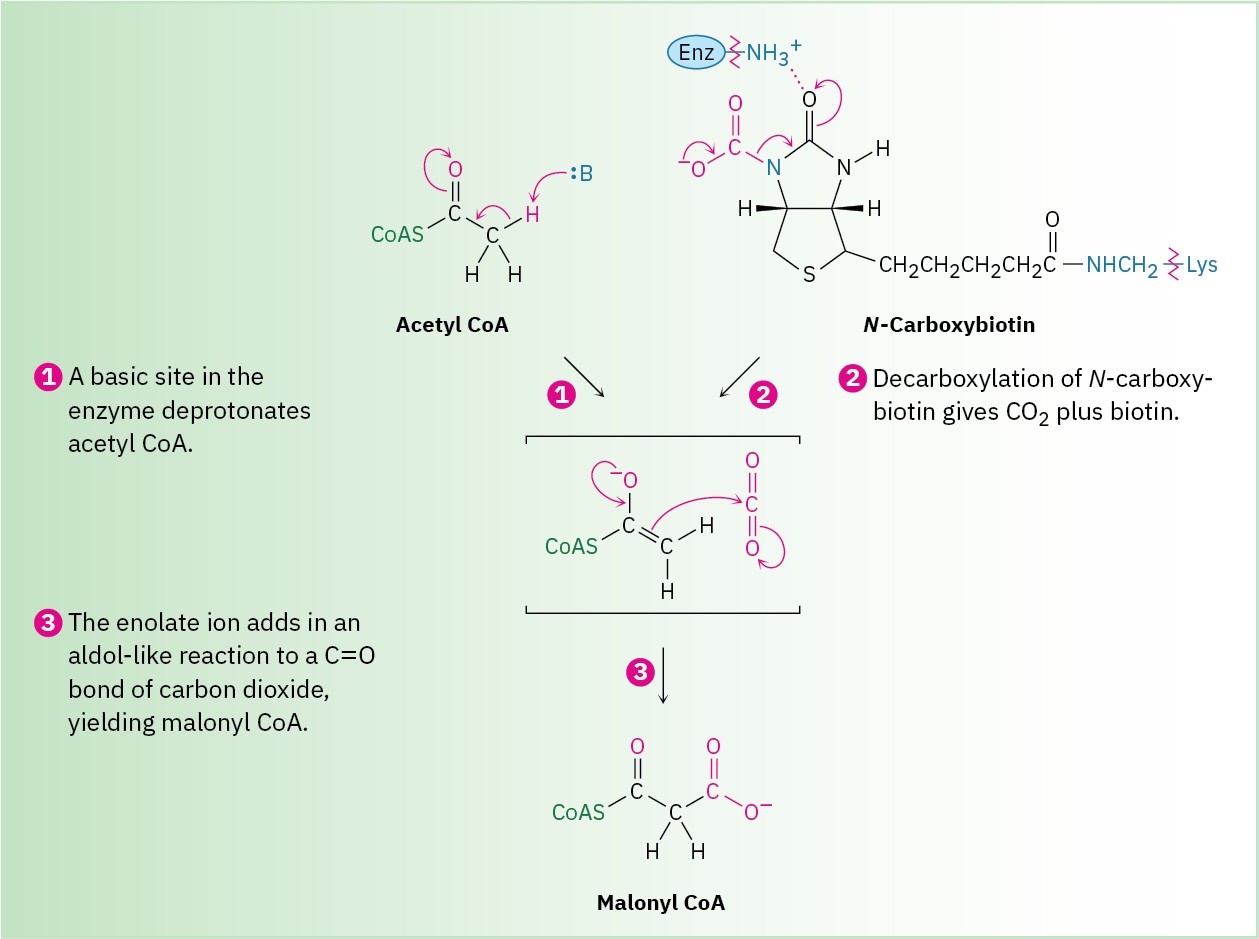
Step 3 is a loading reaction in which acetyl CoA is carboxylated by reaction with HCO3– and ATP to yield malonyl CoA plus ADP. This step requires the coenzyme biotin, which is bonded to the lysine residue of acetyl CoA carboxylase and acts as a carrier of CO2. Biotin first reacts with bicarbonate ion to give N-carboxybiotin, which then reacts with the enolate ion of acetyl CoA and transfers the CO2 group. Thus, biotin acts as a carrier of CO2, binding it in one step and releasing it in another.
The mechanism of the CO2 transfer reaction with acetyl CoA to give malonyl CoA is thought to involve CO2 as the reactive species. One proposal is that the loss of CO2 is favored by hydrogen-bond formation between the N-carboxybiotin carbonyl group and a nearby acidic
site in the enzyme. Simultaneous deprotonation of acetyl CoA by a basic site in the enzyme gives a thioester enolate ion that can react with CO2 as it forms (Figure 29.7).
Figure 29.7 MECHANISM
Mechanism of step 3 in Figure 29.6, the biotin-dependent carboxylation of acetyl CoA to yield malonyl CoA.
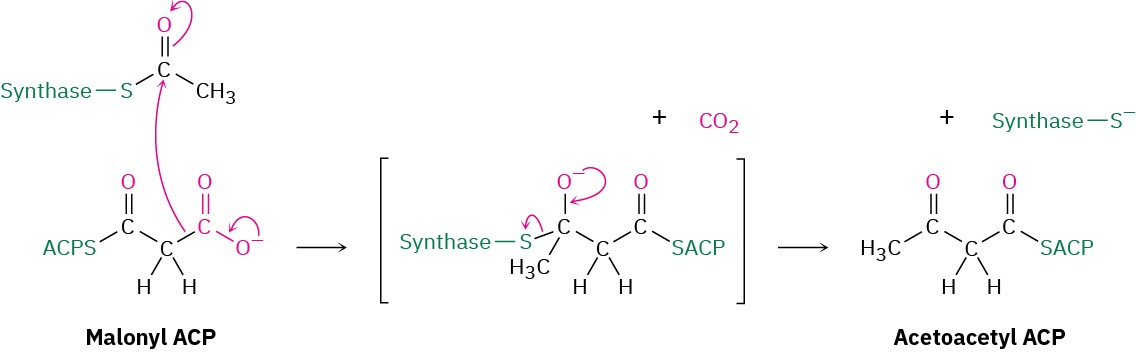
Following the formation of malonyl CoA, another nucleophilic acyl substitution reaction occurs in step 4 to form the more reactive malonyl ACP, thereby binding the malonyl group to an ACP arm of the multienzyme synthase. At this point, both acetyl and malonyl groups are bound to the enzyme, and the stage is set for their condensation.
Step 5 of Figure 29.6: Condensation
The key carbon–carbon bond-forming reaction that builds the fatty-acid chain occurs in step 5. This step is simply a Claisen condensation between acetyl synthase as the electrophilic acceptor and malonyl ACP as the nucleophilic donor. The mechanism of the condensation is thought to involve decarboxylation of malonyl ACP to give an enolate ion, followed by immediate nucleophilic addition of the enolate ion to the carbonyl group of acetyl synthase. Breakdown of the tetrahedral intermediate then gives the four-carbon
condensation product acetoacetyl ACP and frees the synthase binding site for attachment of the chain-elongated acyl group at the end of the sequence.
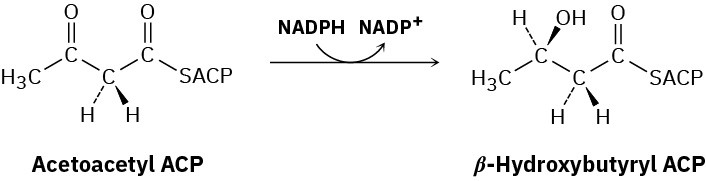
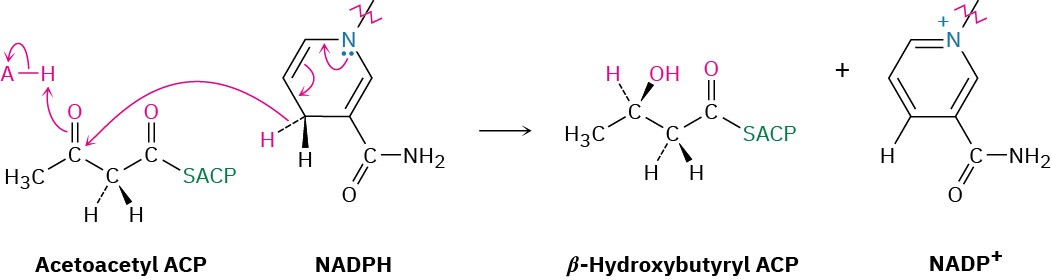
Steps 6–8 of Figure 29.6: Reduction and Dehydration
The ketone carbonyl group in acetoacetyl ACP is next reduced to the alcohol β– hydroxybutyryl ACP by β-keto thioester reductase and NADPH, a reducing coenzyme closely related to NADH. R stereochemistry results at the newly formed chirality center in the β-hydroxy thioester product. (Note that the systematic name of a butyryl group is butanoyl.)
Subsequent dehydration of β-hydroxybutyryl ACP by an E1cB reaction in step 7 yields trans-crotonyl ACP, and the carbon–carbon double bond of crotonyl ACP is reduced by NADPH in step 8 to yield butyryl ACP. The double-bond reduction occurs by conjugate nucleophilic addition of a hydride ion from NADPH to the β carbon of trans-crotonyl ACP. In vertebrates, this reduction occurs by an overall syn addition, but other organisms carry out similar chemistry with different stereochemistry.
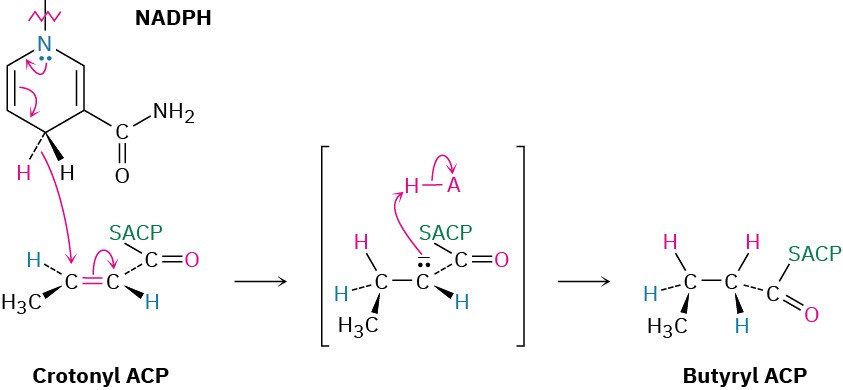
The net effect of the eight steps in the fatty-acid biosynthesis pathway is to take two 2- carbon acetyl groups and combine them into a 4-carbon butyryl group. Further condensation of the butyryl group with another malonyl ACP yields a 6-carbon unit, and still further repetitions add two carbon atoms at a time until the 16-carbon palmitoyl ACP is produced.
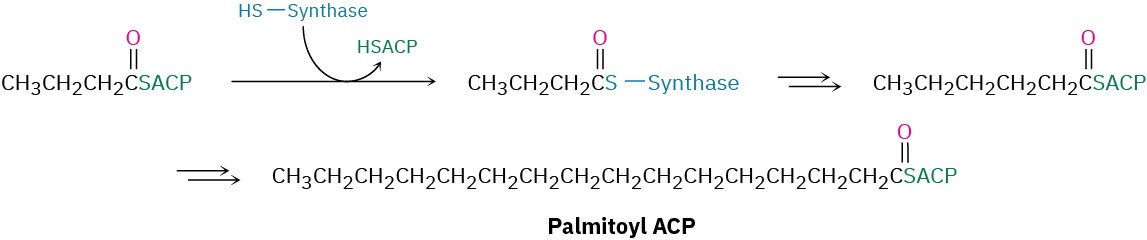
Further chain elongation of palmitic acid occurs by reactions similar to those just described, but CoA rather than ACP acts as the carrier group, and separate enzymes are needed for each step rather than a multienzyme synthase complex.
Problem 29-4
Write a mechanism for the dehydration reaction of β-hydroxybutyryl ACP to yield crotonyl ACP in step 7 of fatty-acid synthesis.
Problem 29-5
Evidence for the role of acetate in fatty-acid biosynthesis comes from isotope-labeling experiments. If acetate labeled with 13C in the methyl group (13CH3CO2H) were incorporated into fatty acids, at what positions in the fatty-acid chain would you expect the 13C label to appear?
Problem 29-6
Does the reduction of acetoacetyl ACP in step 6 occur on the Re face or the Si face of the molecule?