26.5 Amino Acid Analysis of Peptides
To determine the structure of a protein or peptide, we need to answer three questions: What amino acids are present? How much of each is present? In what sequence do the amino acids occur in the peptide chain? The answers to the first two questions are provided by an automated instrument called an amino acid analyzer.
An amino acid analyzer is based on techniques worked out in the 1950s by William Stein and Stanford Moore at the Rockefeller University, who shared the 1972 Nobel Prize in Chemistry for their work. In preparation for analysis, the peptide is broken into its constituent amino acids by reducing all disulfide bonds, capping the –SH groups of cysteine residues by SN2 reaction with iodoacetic acid, and hydrolyzing the amide bonds by heating with aqueous 6 M HCl at 110 °C for 24 hours. The resultant amino acid mixture is then separated into its components by a technique called chromatography, either high-pressure liquid chromatography (HPLC) or ion-exchange chromatography.
In both HPLC and ion-exchange chromatography, the mixture to be separated is dissolved in a solvent, called the mobile phase, and passed through a metal tube or glass column that contains an adsorbent material, called the stationary phase. Because different compounds adsorb to the stationary phase to different extents, they migrate through the chromatography column at different rates and are separated as they emerge (elute) from the end.
In the ion-exchange technique, separated amino acids eluting from the chromatography column mix with a solution of a substance called ninhydrin and undergo a rapid reaction that produces an intense purple color. The color is measured by a spectrometer, and a plot of elution time versus spectrometer absorbance is obtained.
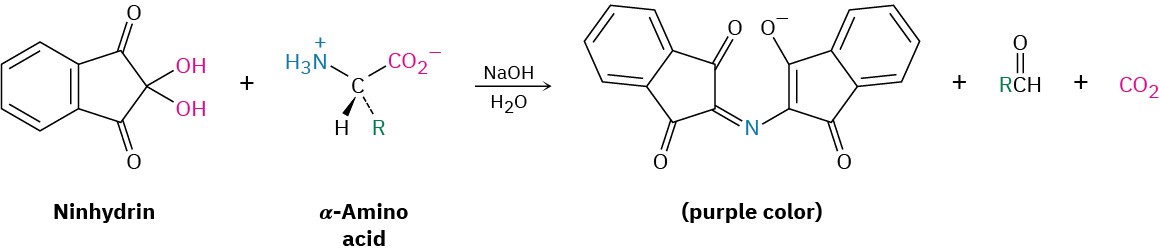
Because the time required for a given amino acid to elute from a standard column is reproducible, the identities of the amino acids in a peptide can be determined. The amount of each amino acid in the sample is determined by measuring the intensity of the purple color resulting from its reaction with ninhydrin. Figure 26.4 shows the results of amino acid analysis of a standard equimolar mixture of 17 α-amino acids. Typically, amino acid analysis requires about 100 picomoles (2–3 μg) of sample for a protein containing about 200 residues.
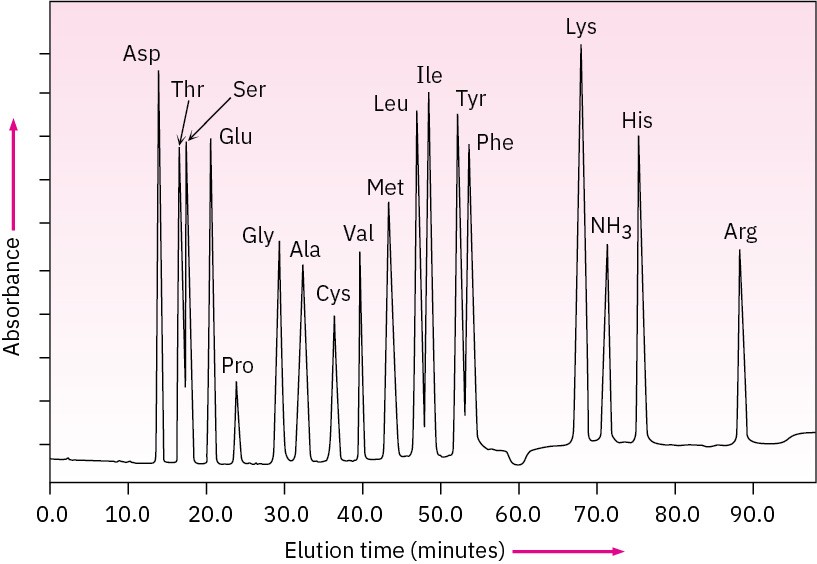
Figure 26.4 Amino acid analysis of an equimolar mixture of 17 amino acids. Problem 26-10
Show the structure of the product you would expect to obtain by SN2 reaction of a cysteine residue with iodoacetic acid.
Problem 26-11
Show the structures of the products obtained on reaction of valine with ninhydrin.

