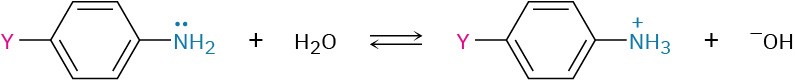
24.4 Basicity of Arylamines
As noted previously, arylamines are generally less basic than alkylamines. Anilinium ion has pKa = 4.63, for instance, whereas methylammonium ion has pKa = 10.64. Arylamines are less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring’s π electron system and are less available for bonding to H+. In resonance terms, arylamines are stabilized relative to alkylamines because of their five resonance forms.
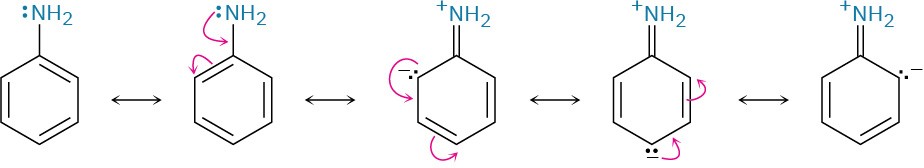
Much of the resonance stabilization is lost on protonation, however, so the energy difference between protonated and nonprotonated forms is higher for arylamines than it is for alkylamines. As a result, arylamines are less basic. Figure 24.5 illustrates this difference.
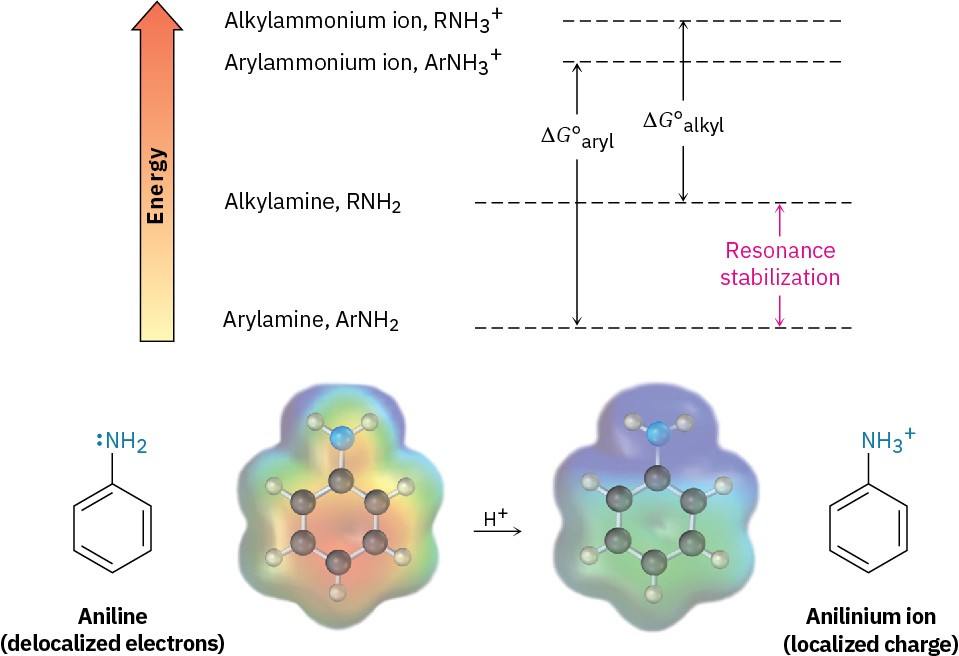
Figure 24.5 Arylamines have a larger positive ∆G° for protonation and are therefore less basic than alkylamines, primarily because of resonance stabilization of the ground state. Electrostatic potential maps show that lone-pair electron density is delocalized in the amine but the charge is localized in the corresponding ammonium ion.
Substituted arylamines can be either more basic or less basic than aniline, depending on the substituent. Electron-donating substituents, such as –CH3, –NH2, and –OCH3, which increase the reactivity of an aromatic ring toward electrophilic substitution (Section 16.4),
also increase the basicity of the corresponding arylamine. Electron-withdrawing substituents, such as –Cl, –NO2, and –CN, which decrease ring reactivity toward electrophilic substitution, also decrease arylamine basicity. Table 24.2 considers only p– substituted anilines, but similar trends are observed for ortho and meta derivatives.
Table 24.2 Base Strength of Some p-Substituted Anilines
|
|
|||
|
|
pKa |
|
|
|
|
–NH2 |
6.15 |
Activating groups |
|
–OCH3 |
5.34 |
||
|
–CH3 |
5.08 |
||
|
–H |
4.63 |
|
|
|
–Cl |
3.98 |
Deactivating groups |
|
|
–Br |
3.86 |
||
|
–CN |
1.74 |
||
|
–NO2 |
1.00 |
||
Problem 24-6
Without looking at Table 24.2, rank the following compounds in order of ascending basicity.
(a)
p-Nitroaniline, p-aminobenzaldehyde, p-bromoaniline (b)
p-Chloroaniline, p-aminoacetophenone, p-methylaniline (c)
p-(Trifluoromethyl)aniline, p-methylaniline, p-(fluoromethyl)aniline