14 Why This Chapter?

Figure 14.1 Saffron, derived from stigmas of the saffron crocus, is the world’s most expensive spice. Its color is caused by the presence of alternating single and double bonds. (credit: modification of work “Welcome to My World! ~ Huron River Watershed” by j van cise photos/Flickr, CC BY 2.0)
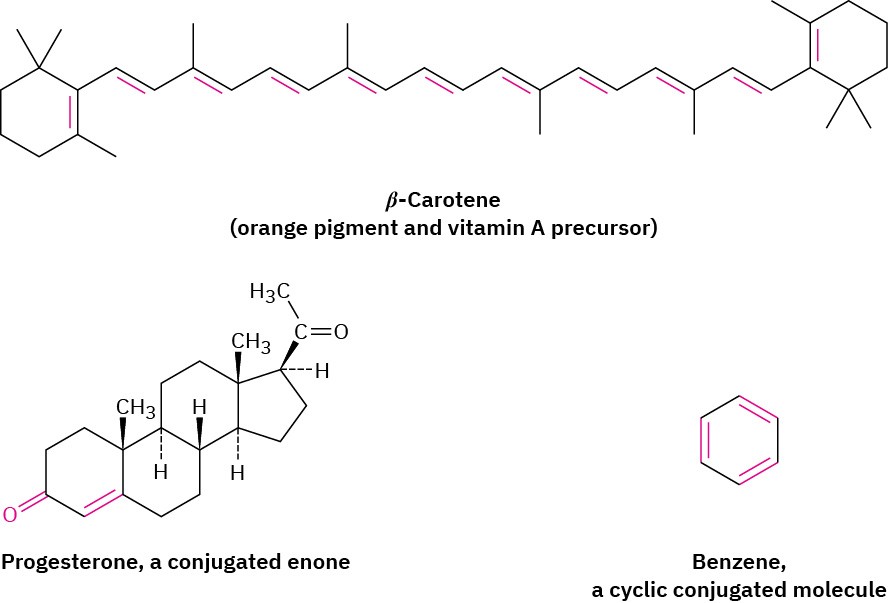
Conjugated compounds of many different sorts are common in nature. Many of the pigments responsible for the brilliant colors of fruits, flowers, and even animals have numerous alternating single and double bonds. β-Carotene, for instance, the orange pigment responsible for the color of carrots and an important source of vitamin A, is a conjugated polyene with 11 double bonds. Conjugated enones (alkene + ketone) are common structural features of many biologically important molecules such as progesterone, the hormone that prepares the uterus for implantation of a fertilized ovum. Cyclic conjugated molecules such as benzene are a major field of study in themselves. In this chapter, we’ll look at some of the distinctive properties of conjugated molecules and at the reasons for those properties.
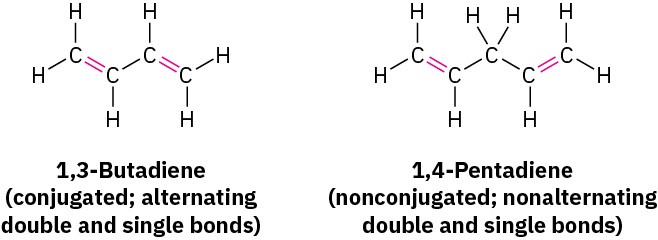
Most of the unsaturated compounds we looked at in the chapters on Alkenes: Structure and Reactivity and Alkenes: Reactions and Synthesis had only one double bond, but many compounds have numerous sites of unsaturation. If the different unsaturations are well separated in a molecule, they react independently, but if they’re close together, they may interact. In particular, compounds that have alternating single and double bonds—so- called conjugated compounds—have some distinctive characteristics. The conjugated diene 1,3-butadiene, for instance, has some properties quite different from those of the nonconjugated 1,4-pentadiene.