13.11 Characteristics of 13C NMR Spectroscopy
At its simplest, 13C NMR makes it possible to count the number of different carbon atoms in a molecule. Look at the 13C NMR spectra of methyl acetate and 1-pentanol shown previously in Figure 13.4b and Figure 13.17b. In each case, a single sharp resonance line is observed for each different carbon atom.
Most 13C resonances are between 0 and 220 ppm downfield from the TMS reference line, with the exact chemical shift of each 13C resonance dependent on that carbon’s electronic environment within the molecule. Figure 13.18 shows the correlation of chemical shift with environment.
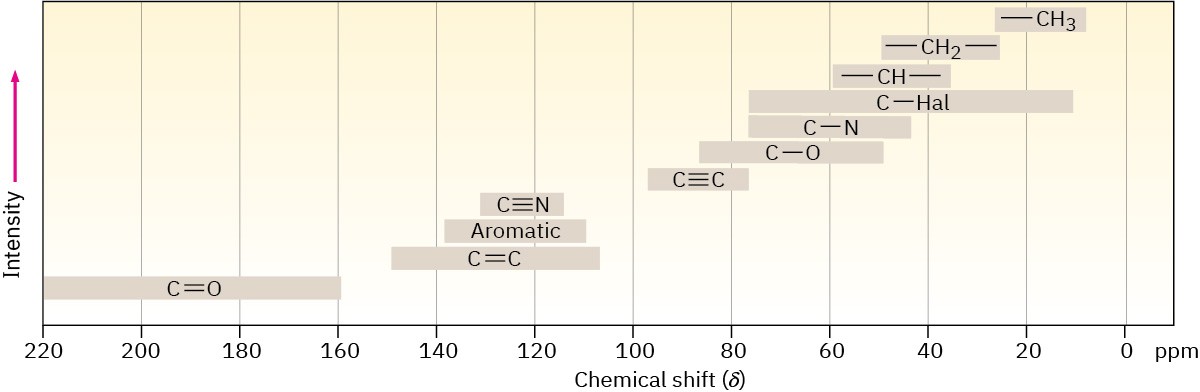
Figure 13.18Chemical shift correlations for 13C NMR.
The factors that determine chemical shifts are complex, but it’s possible to make some generalizations from the data in Figure 13.18. One trend is that a carbon atom’s chemical shift is affected by the electronegativity of nearby atoms. Carbons bonded to oxygen, nitrogen, or halogen absorb downfield (to the left) of typical alkane carbons. Because electronegative atoms attract electrons, they pull electrons away from neighboring carbon atoms, causing those carbons to be deshielded and to come into resonance at a lower field.
Another trend is that sp3-hybridized carbons generally absorb from 0 to 90 δ, while sp2 carbons absorb from 110 to 220 δ. Carbonyl carbons (C=O) are particularly distinct in 13C NMR and are always found at the low-field end of the spectrum, from 160 to 220 δ. Figure
13.19 shows the 13C NMR spectra of 2-butanone and para-bromoacetophenone and indicates the peak assignments. Note that the C=O carbons are at the left edge of the spectrum in each case.
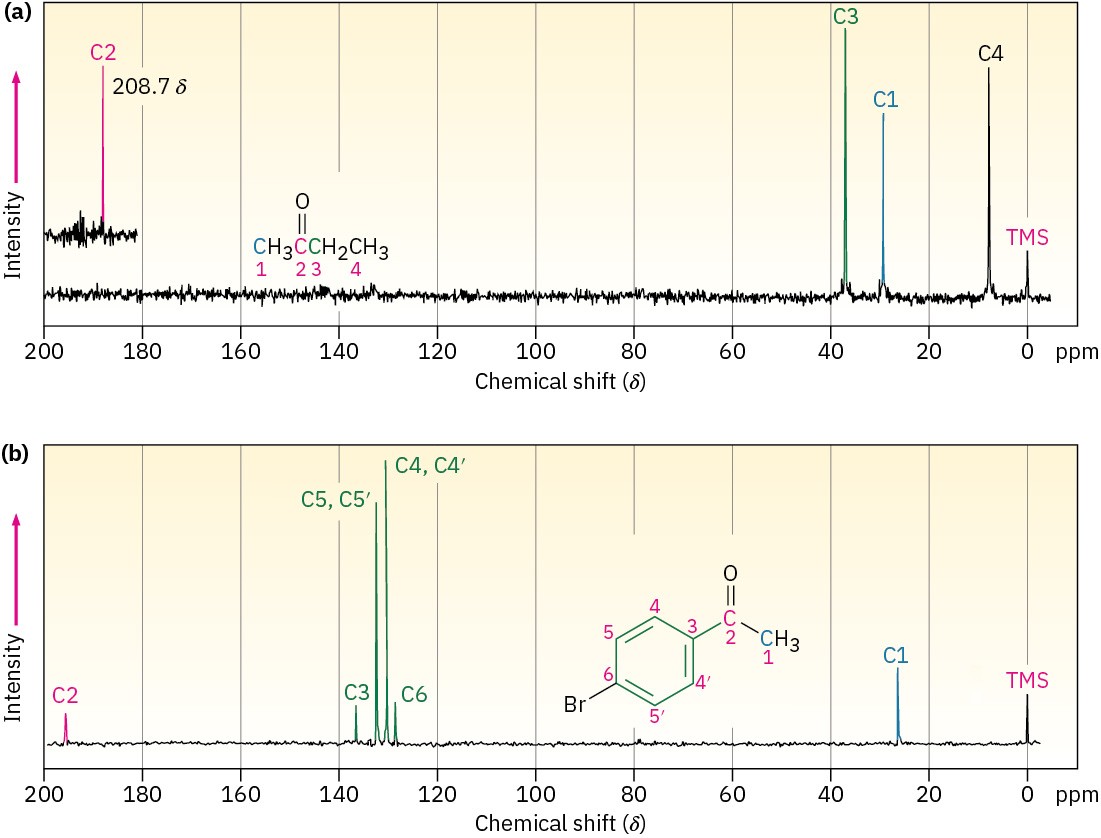
Figure 13.19Carbon-13 NMR spectra of (a) 2-butanone and (b) para– bromoacetophenone.
The 13C NMR spectrum of para-bromoacetophenone is interesting in several ways. Note particularly that only six carbon absorptions are observed, even though the molecule contains eight carbons. para-bromoacetophenone has a symmetry plane that makes ring carbons 4 and 4′, and ring carbons 5 and 5′ equivalent. (Remember from Section 2.4 that aromatic rings have two resonance forms.) Thus, the six ring carbons show only four absorptions in the range 128 to 137 δ.
A second interesting point about both spectra in Figure 13.19 is that the peaks aren’t uniform in size. Some peaks are larger than others even though they are one-carbon resonances (except for the two 2-carbon peaks of para-bromoacetophenone). This difference in peak size is a general feature of broadband-decoupled 13C NMR spectra, and explains why we can’t integrate 13C NMR spectra in the same way we integrate the resonances in a 1H NMR spectrum. The local environment of each carbon atom determines not only its chemical shift but also the time it takes for the nuclei to return to their equilibrium state after receiving a pulse of rf radiation and flipping their spins. Quaternary
carbons, regardless of their hybridization state or substituents, typically give smaller resonances than primary, secondary, or tertiary carbons.
Worked Example 13.3Predicting Chemical Shifts in 13C NMR SpectraAt what approximate positions would you expect ethyl acrylate, H2C═CHCO2CH2CH3, to show 13C NMR absorptions?StrategyIdentify the distinct carbons in the molecule, and note whether each is alkyl, vinylic, aromatic, or in a carbonyl group. Then predict where each absorbs, using Figure 13.18 as necessary.SolutionEthyl acrylate has five chemically distinct carbons: two different C=C, one C=O, one O–C, and one alkyl C. From Figure 13.18, the likely absorptions are
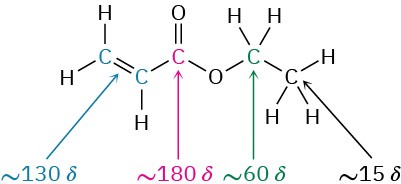
The actual absorptions are at 14.1, 60.5, 128.5, 130.3, and 166.0 δ. Problem 13-17
Predict the number of carbon resonance lines you would expect in the 13C NMR spectra of
the following compounds: (a)
Methylcyclopentane (b)
- Methylcyclohexene (c)
1,2-Dimethylbenzene (d)
- Methyl-2-butene (e)
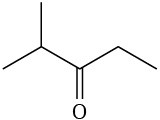
(f)
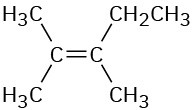
Problem 13-18
Propose structures for compounds that fit the following descriptions: (a)
A hydrocarbon with seven lines in its 13C NMR spectrum (b)
A six-carbon compound with only five lines in its 13C NMR spectrum (c)
A four-carbon compound with three lines in its 13C NMR spectrum Problem 13-19
Classify the resonances in the 13C NMR spectrum of methyl propanoate, CH3CH2CO2CH3 (Figure 13.20).
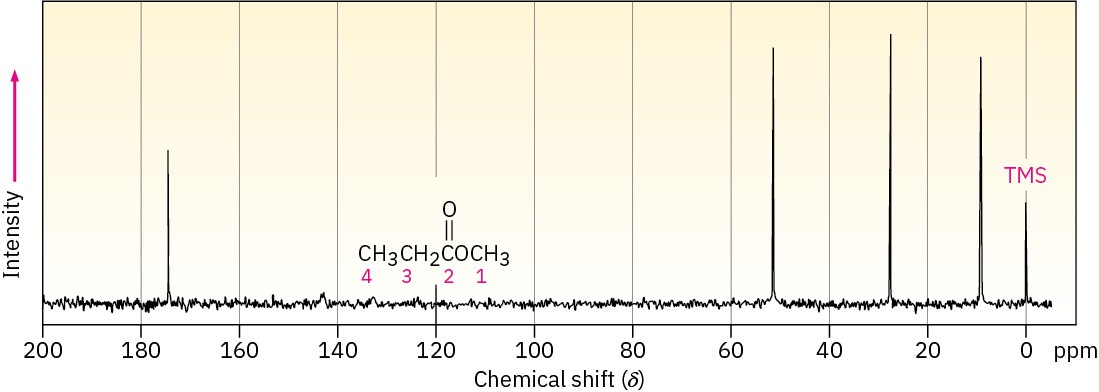
Figure 13.20 13C NMR spectrum of methyl propanoate, Problem 19.

