Additional Problems 11
Visualizing Chemistry
Problem 11-21
Write the product you would expect from reaction of each of the following alkyl halides with (1) Na+ –SCH3 and (2) Na+ –OH (green = Cl):
(a)
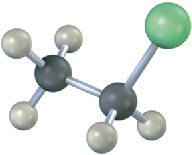
(b)
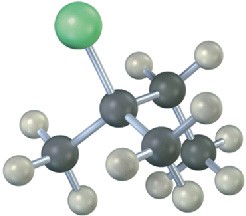
(c)
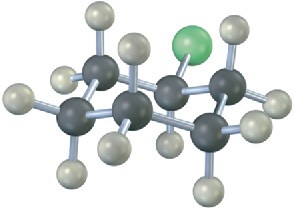
Problem 11-22
From what alkyl bromide was the following alkyl acetate made by SN2 reaction? Write the reaction, showing all stereochemistry.
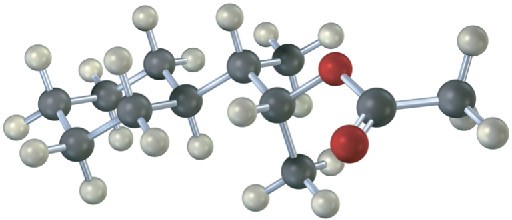
Problem 11-23
Assign R or S configuration to the following molecule, write the product you would expect from SN2 reaction with NaCN, and assign R or S configuration to the product (green = Cl):
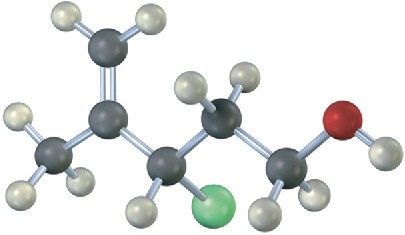
Problem 11-24
Draw the structure and assign Z or E stereochemistry to the product you expect from E2 reaction of the following molecule with NaOH (green = Cl):
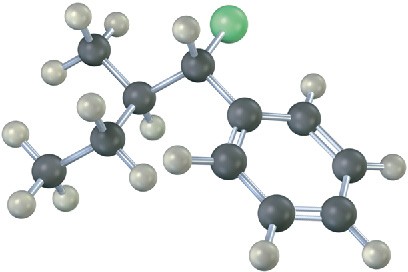
Mechanism Problems
Problem 11-25
Predict the product(s) and show the mechanism for each of the following reactions. What do the mechanisms have in common? Why?
(a)
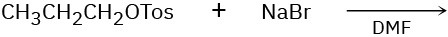
(b)
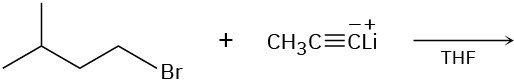
(c)
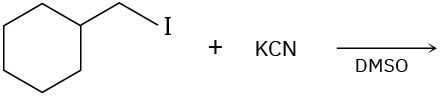
(d)
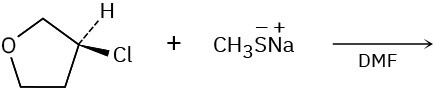
Problem 11-26
Show the mechanism for each of the following reactions. What do the mechanisms have in common? Why?
(a)
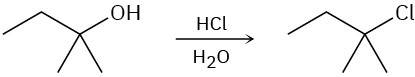
(b)
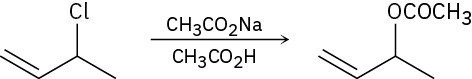
(c)
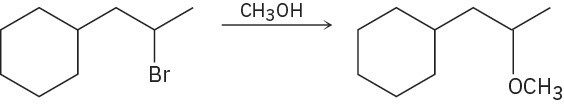
Problem 11-27
Predict the product(s) for each of the following elimination reactions. In each case show the mechanism. What do the mechanisms have in common? Why?
(a)
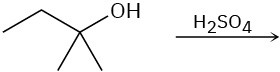
(b)
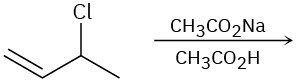
(c)
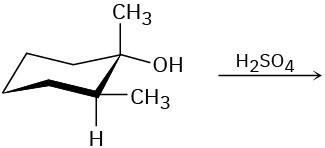
Problem 11-28
Predict the product(s) for each of the following elimination reactions. In each case show the mechanism. What do the mechanisms have in common? Why?
(a)
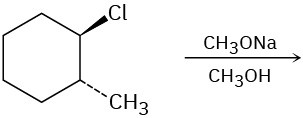
(b)
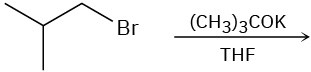
(c)
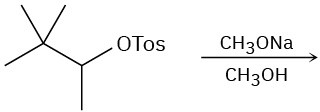
Problem 11-29
Predict the product(s) for each of the following elimination reactions. In each case show the mechanism. What do the mechanisms have in common? Why?
(a)
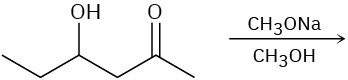
(b)
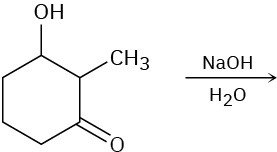
(c)
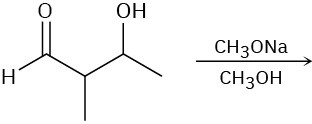
Problem 11-30
Predict the product of each of the following reactions, and indicate if the mechanism is likely to be SN1, SN2, E1, E2, or E1cB.
(a)
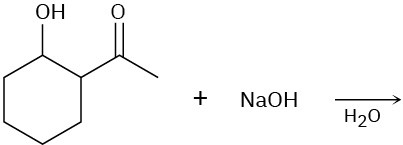
(b)
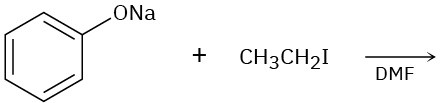
(c)
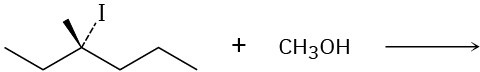
(d)
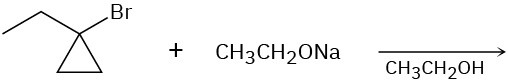
Problem 11-31
We saw in Section 8.7 that bromohydrins are converted into epoxides when treated with base. Propose a mechanism, using curved arrows to show the electron flow.
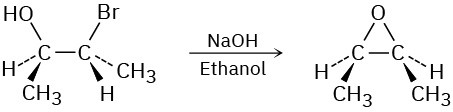
Problem 11-32
The following tertiary alkyl bromide does not undergo a nucleophilic substitution reaction by either SN1 or SN2 mechanisms. Explain.
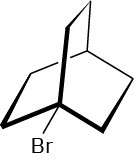
Problem 11-33
Metabolism of S-adenosylhomocysteine (Section 11.6) involves the following sequence. Propose a mechanism for the second step.
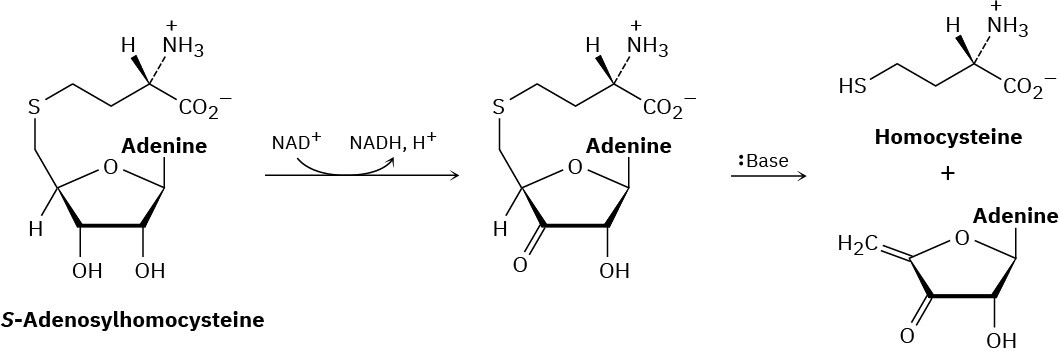
Problem 11-34
Reaction of iodoethane with CN– yields a small amount of isonitrile, CH3CH2N≡C, along with the nitrile CH3CH2C≡N as the major product. Write electron-dot structures for both products, assign formal charges as necessary, and propose mechanisms to account for their formation.
Problem 11-35
One step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.
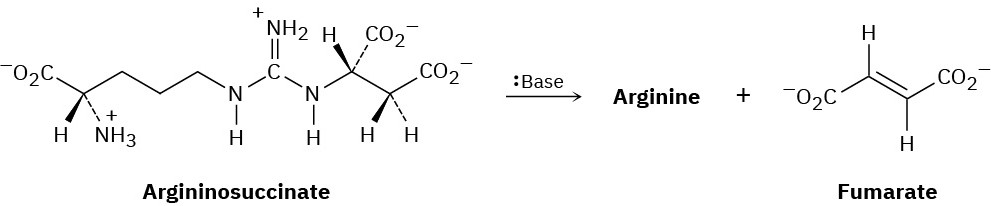
Problem 11-36
Methyl esters (RCO2CH3) undergo a cleavage reaction to yield carboxylate ions plus iodomethane on heating with LiI in dimethylformamide:
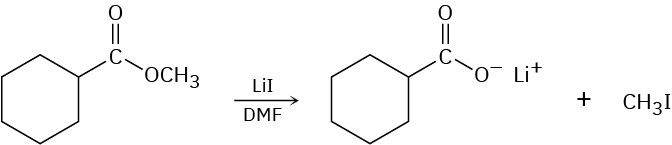
The following evidence has been obtained: (1) The reaction occurs much faster in DMF than in ethanol. (2) The corresponding ethyl ester (RCO2CH2CH3) cleaves approximately 10 times more slowly than the methyl ester. Propose a mechanism for the reaction. What other kinds of experimental evidence could you gather to support your hypothesis?
Problem 11-37
SN2 reactions take place with inversion of configuration, and SN1 reactions take place with racemization. The following substitution reaction, however, occurs with complete retention of configuration. Propose a mechanism. (Hint: two inversions = retention.)
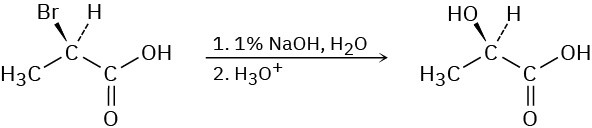
Problem 11-38
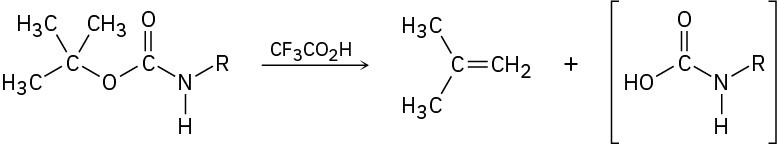
Propose a mechanism for the following reaction, an important step in the laboratory synthesis of proteins:
Nucleophilic Substitution Reactions
Problem 11-39
Draw all isomers of C4H9Br, name them, and arrange them in order of decreasing reactivity in the SN2 reaction.
Problem 11-40
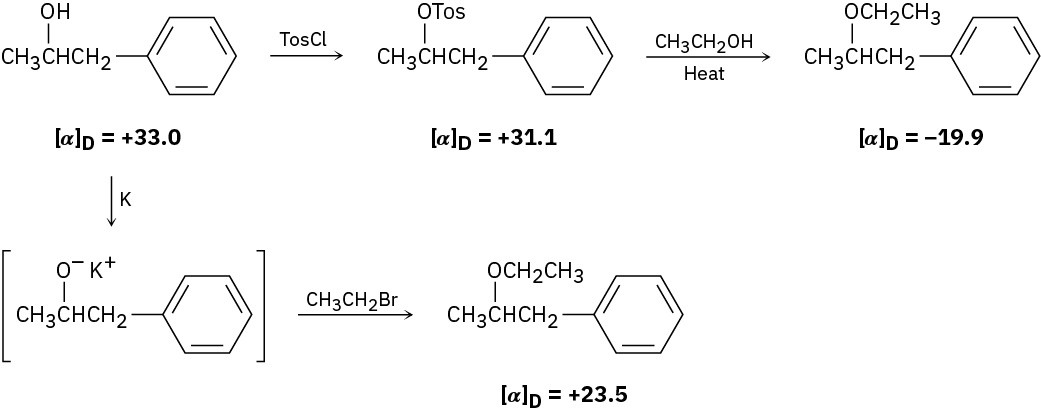
The following Walden cycle has been carried out: Explain the results, and indicate where inversion occurs.
Problem 11-41
Which compound in each of the following pairs will react faster in an SN2 reaction with OH–?
(a) CH3Br or CH3I
(b) CH3CH2I in ethanol or in dimethyl sulfoxide
(c) (CH3)3CCl or CH3Cl
(d) H2C=CHBr or H2C=CHCH2Br
Problem 11-42
Which reactant in each of the following pairs is more nucleophilic? Explain.
(a) –NH2 or NH3
(b) H2O or CH3CO2–
(c) BF3 or F–
(d) (CH3)3P or (CH3)3N
(e) I– or Cl–
(f) –C≡N or –OCH3
Problem 11-43
What effect would you expect the following changes to have on the rate of the SN2 reaction of 1-iodo-2-methylbutane with cyanide ion?
(a) The CN– concentration is halved, and the 1-iodo-2-methylbutane concentration is doubled.
(b) Both the CN– and the 1-iodo-2-methylbutane concentrations are tripled.
Problem 11-44
What effect would you expect the following changes to have on the rate of the reaction of ethanol with 2-iodo-2-methylbutane?
(a) The concentration of the halide is tripled.
(b) The concentration of the ethanol is halved by adding diethyl ether as an inert solvent.
Problem 11-45
How might you prepare each of the following using a nucleophilic substitution reaction at some step?
(a)
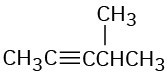
(b)
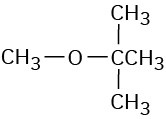
(c)
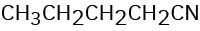
(d)
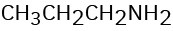
Problem 11-46
Which reaction in each of the following pairs would you expect to be faster?
(a)
The SN2 displacement by I– on CH3Cl or on CH3OTos
(b)
The SN2 displacement by CH3CO2– on bromoethane or on bromocyclohexane
(c)
The SN2 displacement on 2-bromopropane by CH3CH2O– or by CN–
(d)
The SN2 displacement by HC≡C– on bromomethane in benzene or in acetonitrile
Problem 11-47
Predict the product and give the stereochemistry resulting from reaction of each of the following nucleophiles with (R)-2-bromooctane:
(a) –CN
(b) CH3CO2–
(c) CH3S–
Problem 11-48
(R)-2-Bromooctane undergoes racemization to give (±)-2-bromooctane when treated with NaBr in dimethyl sulfoxide. Explain.
Elimination Reactions
Problem 11-49
Propose structures for compounds that fit the following descriptions:
(a)
An alkyl halide that gives a mixture of three alkenes on E2 reaction
(b)
An organohalide that will not undergo nucleophilic substitution
(c)
An alkyl halide that gives the non-Zaitsev product on E2 reaction
(d)
An alcohol that reacts rapidly with HCl at 0 °C
Problem 11-50
What products would you expect from the reaction of 1-bromopropane with each of the following?
(a) NaNH2
(b) KOC(CH3)3
(c) NaI
(d) NaCN
(e) NaC≡CH (f)
Mg, then H2O Problem 11-51
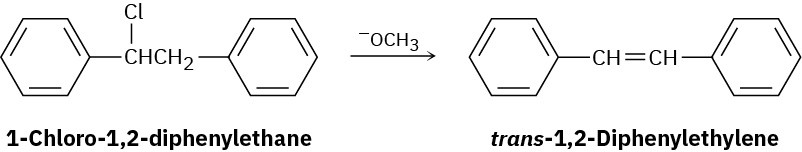
1-Chloro-1,2-diphenylethane can undergo E2 elimination to give either cis– or trans-1,2- diphenylethylene (stilbene). Draw Newman projections of the reactive conformations leading to both possible products, and suggest a reason why the trans alkene is the major product.
Problem 11-52
Predict the major alkene product of the following E1 reaction:
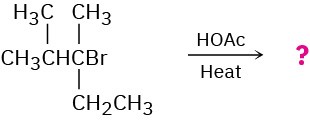
Problem 11-53
There are eight diastereomers of 1,2,3,4,5,6-hexachlorocyclohexane. Draw each in its more stable chair conformation. One isomer loses HCl in an E2 reaction nearly 1000 times more slowly than the others. Which isomer reacts so slowly, and why?
General Problems
Problem 11-54
The following reactions are unlikely to occur as written. Tell what is wrong with each, and predict the actual product.
(a)
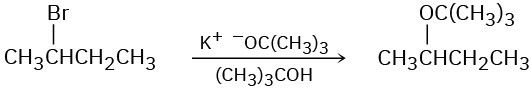
(b)
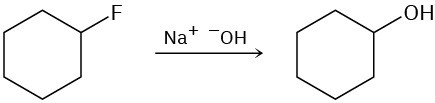
(c)
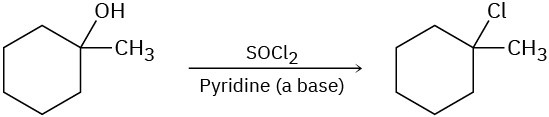
Problem 11-55
Arrange the following carbocations in order of increasing stability.
(a)
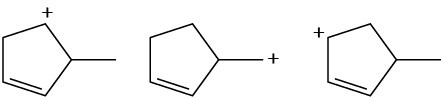
(b)
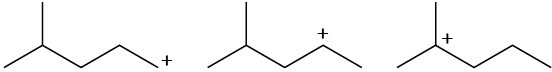
(c)
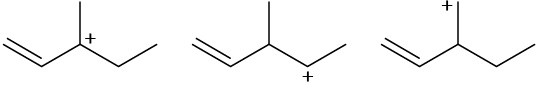
Problem 11-56
Order each of the following sets of compounds with respect to SN1 reactivity:
(a)
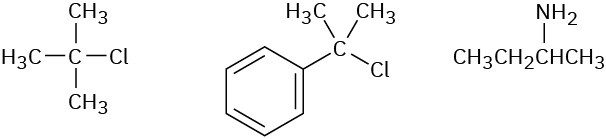
(b)
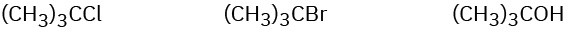
(c)
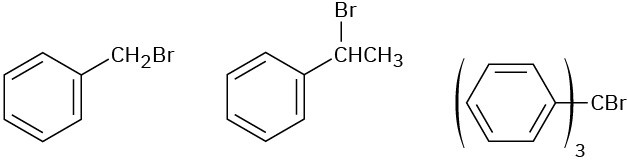
Problem 11-57
Order each of the following sets of compounds with respect to SN2 reactivity:
(a)
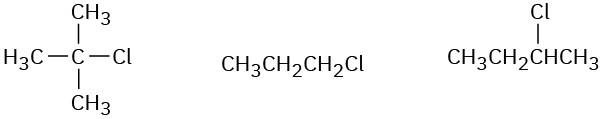
(b)
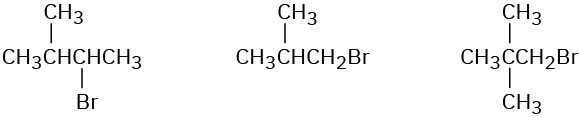
(c)
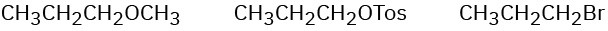
Problem 11-58
Predict the major product(s) of each of the following reactions. Identify those reactions where you would expect the product mixture to rotate plane-polarized light.
(a)
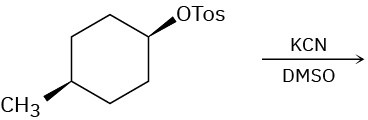
(b)
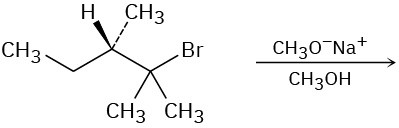
(c)
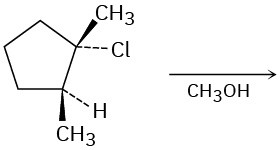
Problem 11-59
Reaction of the following S tosylate with cyanide ion yields a nitrile product that also has S
stereochemistry. Explain.
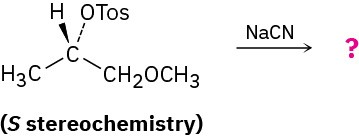
Problem 11-60
Ethers can often be prepared by SN2 reaction of alkoxide ions, RO–, with alkyl halides. Suppose you wanted to prepare cyclohexyl methyl ether. Which of the following two possible routes would you choose? Explain.
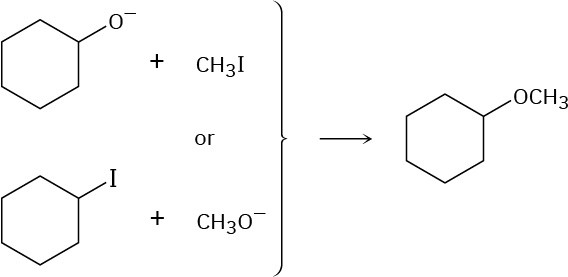
Problem 11-61
How can you explain the fact that trans-1-bromo-2-methylcyclohexane yields the non- Zaitsev elimination product 3-methylcyclohexene on treatment with base?
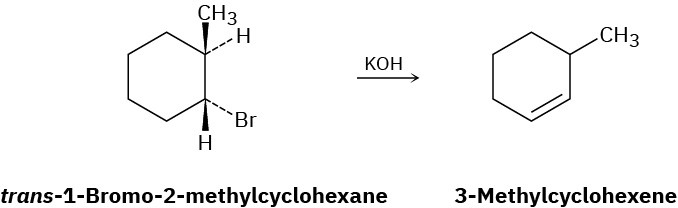
Problem 11-62
Predict the product(s) of the following reaction, indicating stereochemistry where necessary:
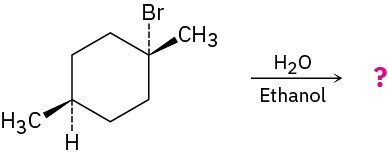
Problem 11-63
Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an E2 process. In studying the stereochemistry of this elimination, it was found that (Z)-2-chloro-2-butenedioic acid reacts 50 times as fast as the corresponding E isomer. What conclusion can you draw about the stereochemistry of eliminations in vinylic halides? How does this result compare with eliminations of alkyl halides?
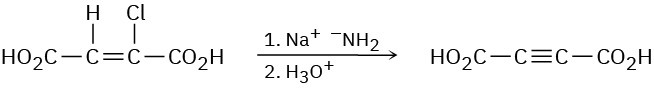
Problem 11-64
Based on your answer to Problem 11-63, predict the product(s) and show the mechanism for each of the following reactions.
(a)
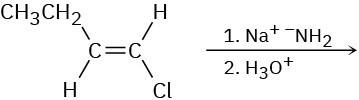
(b)
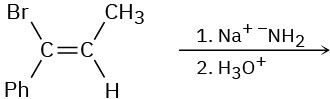
(c)
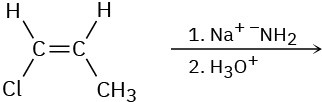
Problem 11-65
(S)-2-Butanol slowly racemizes on standing in dilute sulfuric acid. Explain.
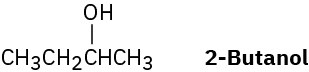
Problem 11-66
Reaction of HBr with (R)-3-methyl-3-hexanol leads to racemic 3-bromo-3-methylhexane. Explain.
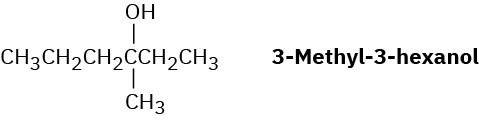
Problem 11-67
Treatment of 1-bromo-2-deuterio-2-phenylethane with strong base leads to a mixture of deuterated and nondeuterated phenylethylenes in an approximately 7 : 1 ratio. Explain.
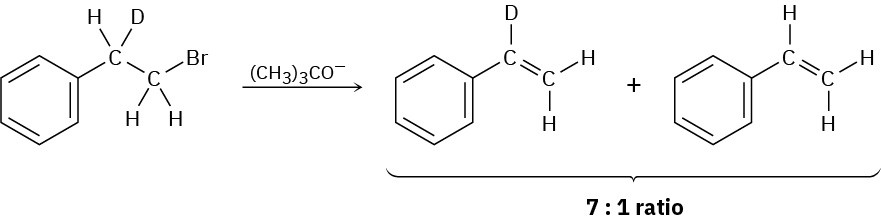
Problem 11-68
Propose a structure for an alkyl halide that gives only (E)-3-methyl-2-phenyl-2-pentene on E2 elimination. Make sure you indicate the stereochemistry.
Problem 11-69
Although anti periplanar geometry is preferred for E2 reactions, it isn’t absolutely necessary. The following deuterated bromo compound reacts with strong base to yield an undeuterated alkene. A syn elimination has occurred. Make a molecular model of the reactant, and explain the result.
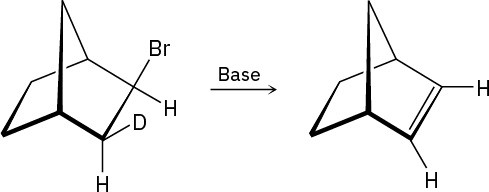
Problem 11-70
The reaction of 1-chlorooctane with CH3CO2– to give octyl acetate is greatly accelerated by adding a small quantity of iodide ion. Explain.
Problem 11-71
Compound X is optically inactive and has the formula C16H16Br2. On treatment with strong base, X gives hydrocarbon Y, C16H14. Compound Y absorbs 2 equivalents of hydrogen when reduced over a palladium catalyst and reacts with ozone to give two fragments. One fragment, Z, is an aldehyde with formula C7H6O. The other fragment is glyoxal, (CHO)2.
Write the reactions involved, and suggest structures for X, Y, and Z. What is the stereochemistry of X?
Problem 11-72
When a primary alcohol is treated with p-toluenesulfonyl chloride at room temperature in the presence of an organic base such as pyridine, a tosylate is formed. When the same reaction is carried out at higher temperature, an alkyl chloride is often formed. Explain.
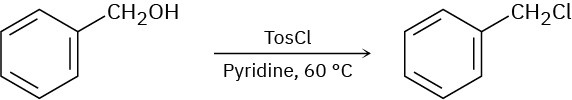
Problem 11-73
The amino acid methionine is formed by a methylation reaction of homocysteine with N– methyltetrahydrofolate. The stereochemistry of the reaction has been probed by carrying out the transformation using a donor with a “chiral methyl group,” which contains protium (H), deuterium (D), and tritium (T) isotopes of hydrogen. Does the methylation reaction occur with inversion or retention of configuration?
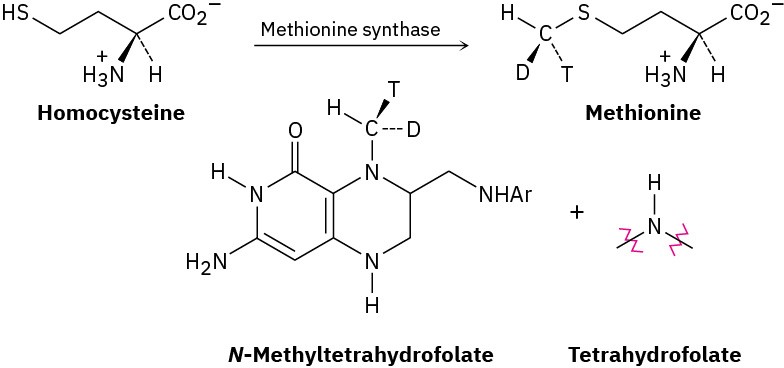
Problem 11-74
Amines are converted into alkenes by a two-step process called the Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.
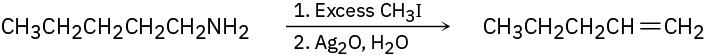
Problem 11-75
The antipsychotic drug flupentixol is prepared by the following scheme:
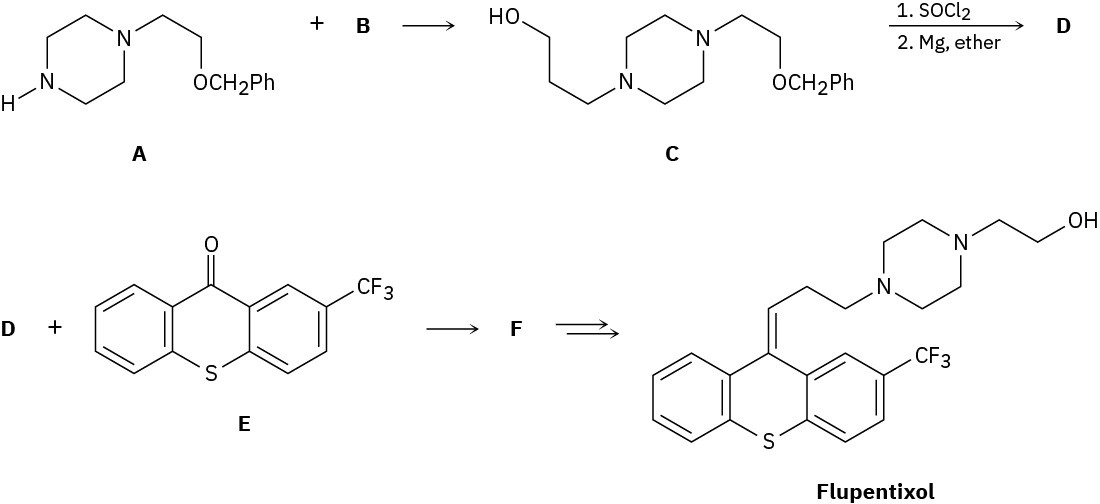
(a) What alkyl chloride B reacts with amine A to form C?
(b) Compound C is treated with SOCl2, and the product is allowed to react with magnesium metal to give a Grignard reagent D. What is the structure of D?
(c) We’ll see in Section 19.7 that Grignard reagents add to ketones, such as E, to give tertiary alcohols, such as F. Because of the newly formed chirality center, compound F exists as a pair of enantiomers. Draw both, and assign R,S configurations.
(d) Two stereoisomers of flupentixol are subsequently formed from F, but only one is shown. Draw the other isomer, and identify the type of stereoisomerism.

