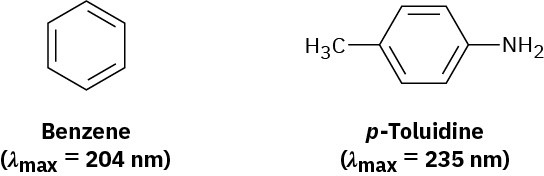14.9 Conjugation, Color, and the Chemistry of Vision
Why are some organic compounds colored while others are not? β-Carotene, the pigment in carrots, is yellow-orange, for instance, while cholesterol is colorless. The answer involves both the chemical structures of colored molecules and the way we perceive light.
The visible region of the electromagnetic spectrum is adjacent to the ultraviolet region, extending from approximately 400 to 800 nm. Colored compounds have such elaborate systems of conjugation that their “UV” absorptions extend into the visible region. β– Carotene, for instance, has 11 double bonds in conjugation, and its absorption occurs at λmax = 455 nm (Figure 14.14).
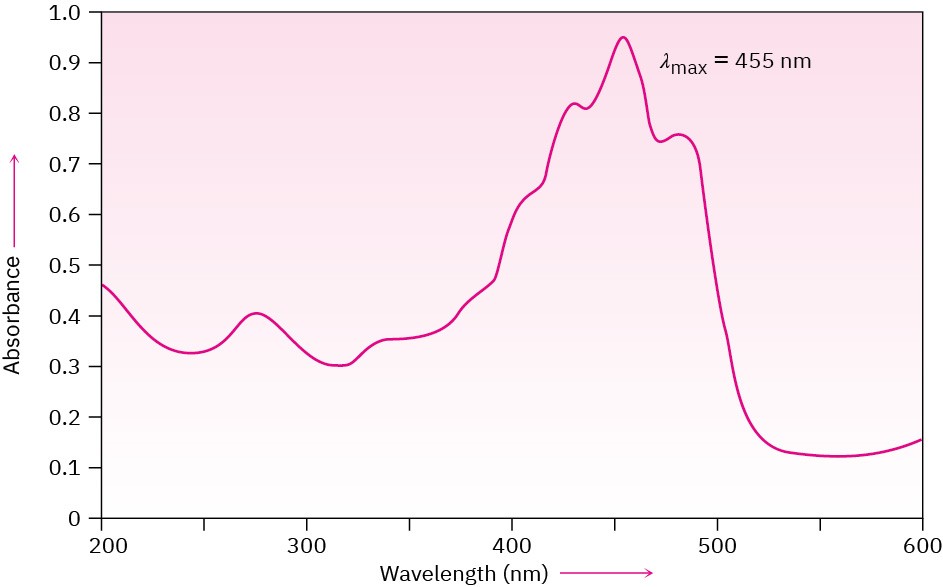
Figure 14.14Ultraviolet spectrum of β-carotene, a conjugated molecule with 11 double bonds. The absorption occurs in the visible region.
“White” light from the sun or from a lamp consists of all wavelengths in the visible region. When white light strikes β-carotene, the wavelengths from 400 to 500 nm (blue) are absorbed while all other wavelengths are transmitted and can reach our eyes. We therefore see the white light with the blue removed, which we perceive as a yellow-orange color for β-carotene.
Conjugation is crucial not only for the colors we see in organic molecules but also for the light-sensitive molecules on which our visual system is based. The key substance for vision is dietary β-carotene, which is converted to vitamin A by enzymes in the liver, oxidized to an aldehyde called 11-trans-retinal, and then isomerized by a change in geometry of the C11–C12 double bond to produce 11-cis-retinal.
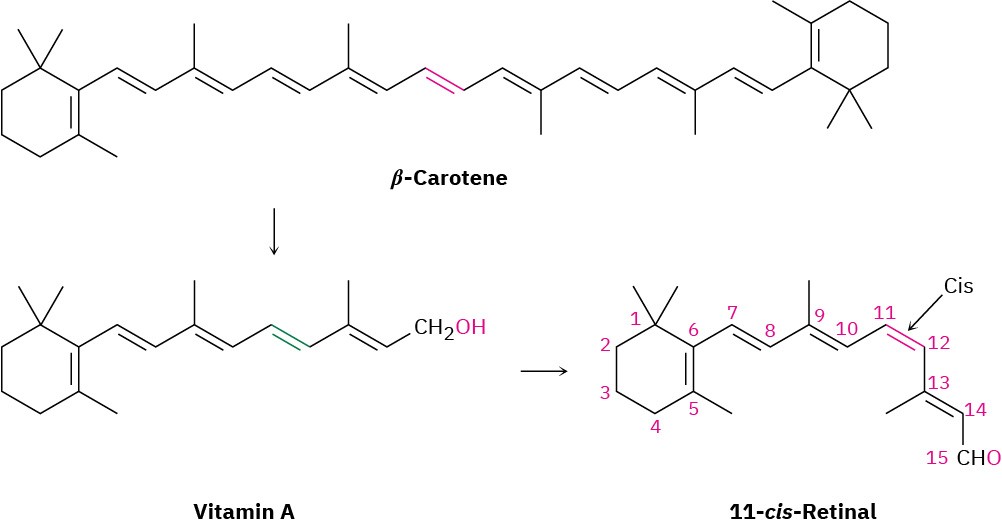
There are two main types of light-sensitive receptor cells in the retina of the human eye: rod cells and cone cells. The 3 million or so rod cells are primarily responsible for seeing dim light and shades of gray, whereas the 100 million cone cells are responsible for seeing bright light and colors. In the rod cells of the eye, 11-cis-retinal is converted into rhodopsin, a light-sensitive substance formed from the protein opsin and 11-cis-retinal. When light strikes the rod cells, isomerization of the C11–C12 double bond occurs and trans– rhodopsin, called metarhodopsin II, is produced. In the absence of light, this cis–trans isomerization takes approximately 1100 years, but in the presence of light, it occurs within 2 × 10–13 seconds! Isomerization of rhodopsin is accompanied by a change in molecular geometry, which in turn causes a nerve impulse to be sent through the optic nerve to the brain, where it is perceived as vision.
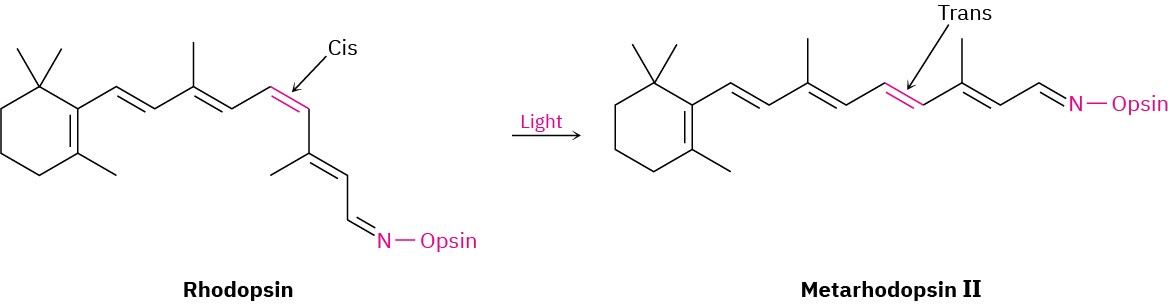
Metarhodopsin II is then recycled back into rhodopsin by a multistep sequence involving cleavage to all-trans-retinal and cis–trans isomerization back to 11-cis-retinal.
Additional Problems 14 • Additional Problems 14 • Additional Problems Visualizing Chemistry Problem 14-16
Show the structures of all possible adducts of the following diene with 1 equivalent of HCl:
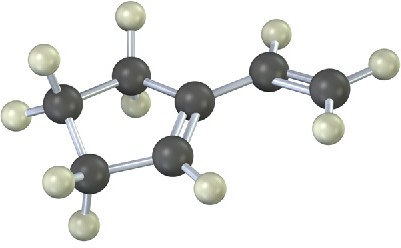
Problem 14-17
Show the product of the Diels–Alder reaction of the following diene with 3-buten-2-one, H2C=CHCOCH3. Make sure you show the full stereochemistry of the reaction product.
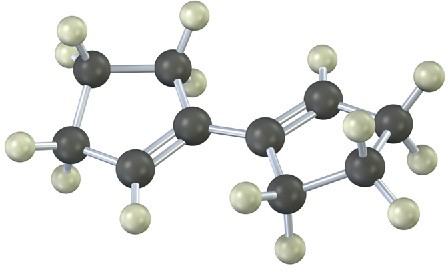
Problem 14-18
The following diene does not undergo Diels–Alder reactions. Explain.
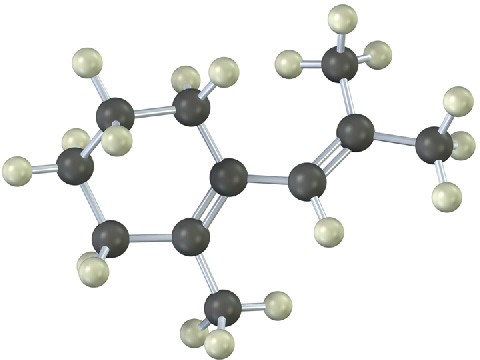
Problem 14-19
The following model is that of an allylic carbocation intermediate formed by protonation of a conjugated diene with HBr. Show the structure of the diene and the structures of the final reaction products.
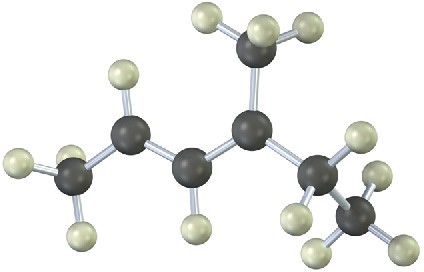
Mechanism Problems
Problem 14-20
Predict the major product(s) from the addition of 1 equivalent of HX and show the mechanism for each of the following reactions.
(a)
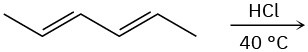
(b)
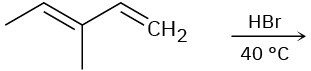
(c)
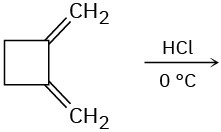
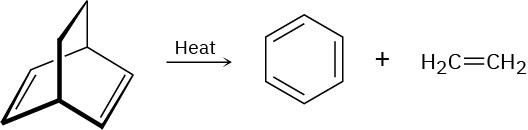
Problem 14-21
We’ve seen that the Diels–Alder cycloaddition reaction is a one-step, pericyclic process that occurs through a cyclic transition state. Propose a mechanism for the following reaction:
Problem 14-22
In light of your answer to Problem 14-21 propose mechanisms for the following reactions. (a)
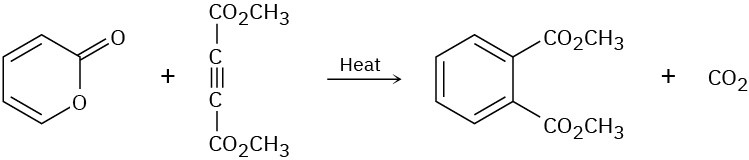
(b)
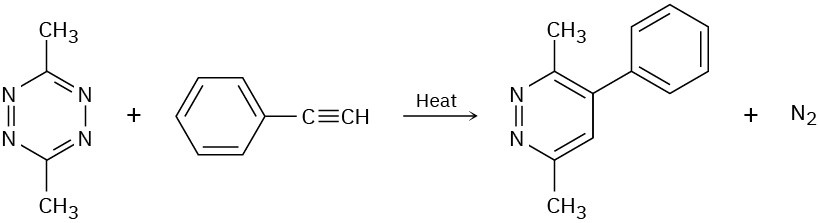
Problem 14-23
Luminol, which is used by forensic scientists to find blood, fluoresces as a result of Diels– Alder-like process. The dianion of luminol reacts with O2 to form an unstable peroxide intermediate that then loses nitrogen to form a dicarboxylate and emit light. The process is similar to that in Problems 14-21 and 14-22. Propose a mechanism for this process.
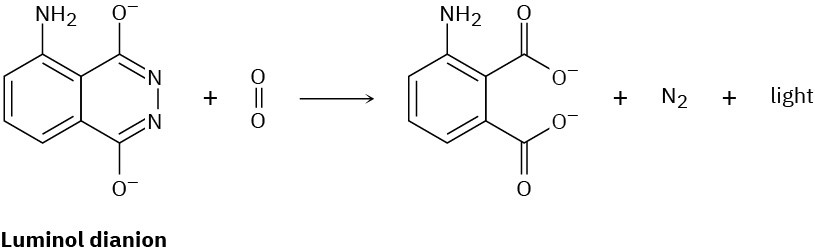
Problem 14-24
A useful diene in the synthesis of many naturally occurring substances is known as Danishefsky’s diene. It’s useful because after the Diels–Alder reaction it can be converted into a product that can’t be accessed by a typical Diels–Alder reaction. Show the Diels– Alder adduct and propose a mechanism that accounts for the final products.
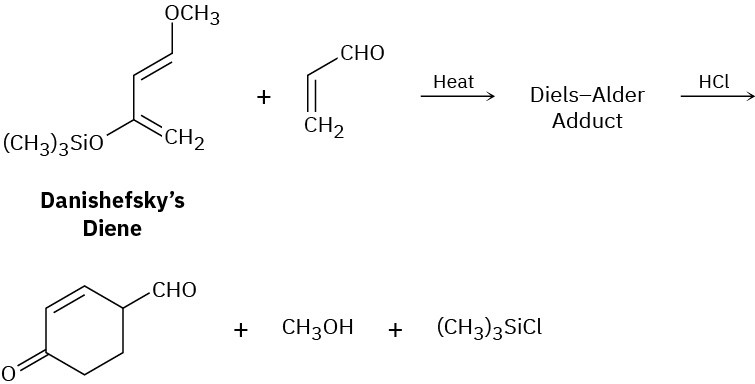
Conjugated Dienes
Problem 14-25
Name the following compounds:
(a)
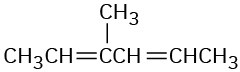
(b)
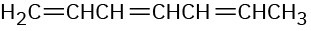
(c)

(d)
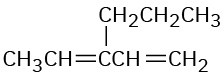
Problem 14-26
Draw and name the six possible diene isomers of formula C5H8. Which of the six are conjugated dienes?
Problem 14-27
What product(s) would you expect to obtain from reaction of 1,3-cyclohexadiene with each of the following?
(a)
1 mol Br2 in CH2Cl2 (b)
O3 followed by Zn (c)
1 mol HCl in ether (d)
1 mol DCl in ether (e)
3-Buten-2-one (H2C=CHCOCH3)
(f)
Excess OsO4, followed by NaHSO3 Problem 14-28
Electrophilic addition of Br2 to isoprene (2-methyl-1,3-butadiene) yields the following product mixture:
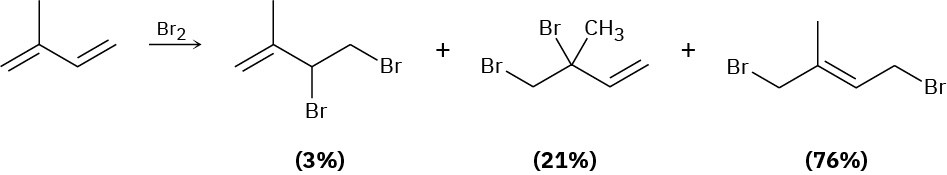
Of the 1,2-addition products, explain why 3,4-dibromo-3-methyl-1-butene (21%) predominates over 3,4-dibromo-2-methyl-1-butene (3%).
Problem 14-29
Propose a structure for a conjugated diene that gives the same product from both 1,2 and 1,4-addition of HBr.
Problem 14-30
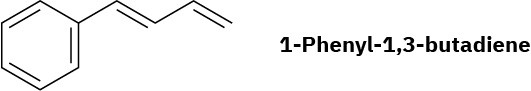
Draw the possible products resulting from addition of 1 equivalent of HCl to 1-phenyl-1,3- butadiene. Which would you expect to predominate, and why?
Diels–Alder Reactions
Problem 14-31
Predict the products of the following Diels–Alder reactions:
(a)
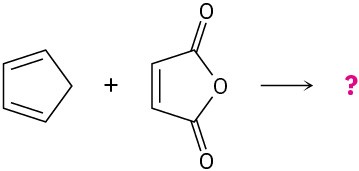
(b)
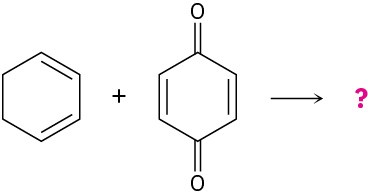
Problem 14-32
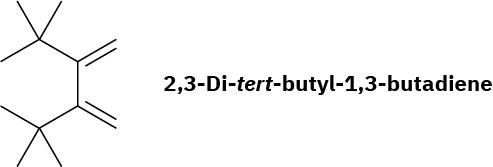
2,3-Di-tert-butyl-1,3-butadiene does not undergo Diels–Alder reactions. Explain.
Problem 14-33
Show the structure, including stereochemistry, of the product from the following Diels– Alder reaction:
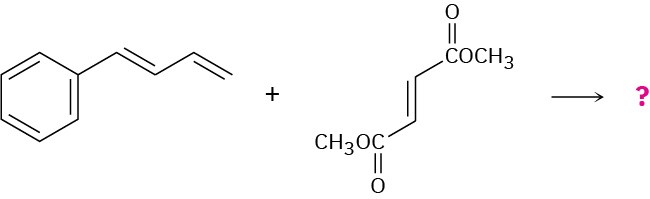
Problem 14-34
How can you account for the fact that cis-1,3-pentadiene is much less reactive than trans– 1,3-pentadiene in the Diels–Alder reaction?
Problem 14-35
Would you expect a conjugated diyne such as 1,3-butadiyne to undergo Diels–Alder reaction with a dienophile? Explain.
Problem 14-36
Reaction of isoprene (2-methyl-1,3-butadiene) with ethyl propenoate gives a mixture of two Diels–Alder adducts. Show the structure of both, and explain why a mixture is formed.
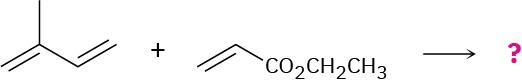
Problem 14-37
Rank the following dienophiles in order of their expected reactivity in the Diels–Alder reaction.
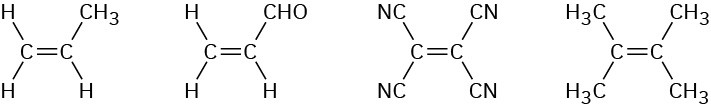
Problem 14-38
1,3-Cyclopentadiene is very reactive in Diels–Alder cycloaddition reactions, but 1,3- cyclohexadiene is less reactive and 1,3-cycloheptadiene is nearly inert. Explain. (Molecular models are helpful.)
Problem 14-39
1,3-Pentadiene is much more reactive in Diels–Alder reactions than 2,4-pentadienal. Why might this be?
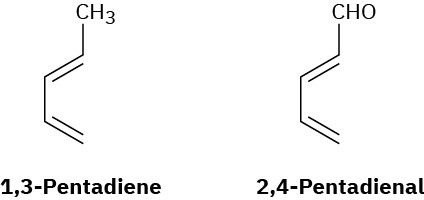
Problem 14-40
How could you use Diels–Alder reactions to prepare the following products? Show the starting diene and dienophile in each case.
(a)
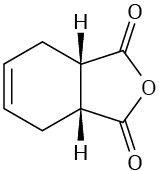
(b)
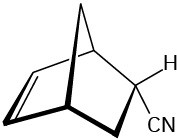
(c)
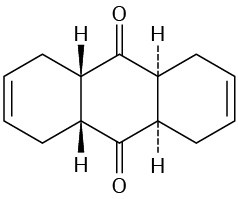
(d)
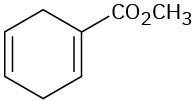
Problem 14-41
Show the product of the following reaction.
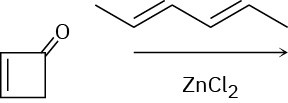
Diene Polymers
Problem 14-42
Tires whose sidewalls are made of natural rubber tend to crack and weather rapidly in areas around cities where high levels of ozone and other industrial pollutants are found. Explain.
Problem 14-43
1,3-Cyclopentadiene polymerizes slowly at room temperature to yield a polymer that has no double bonds except on the ends. On heating, the polymer breaks down to regenerate 1,3-cyclopentadiene. Propose a structure for the product.
UV Spectroscopy
Problem 14-44
Arrange the molecules in each of the following sets according to where you would expect to find their wavelength of maximum absorption in UV spectroscopy, from shortest to longest wavelength.
(a)
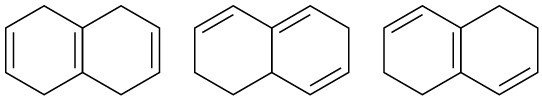
(b)
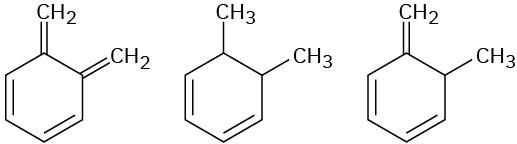
(c)
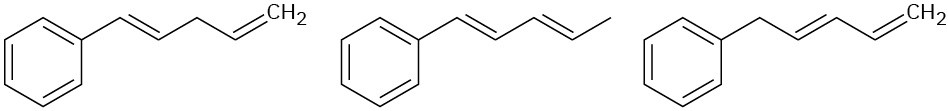
Problem 14-45
Which of the following compounds would you expect to have a π → π* UV absorption in the 200 to 400 nm range?
(a)
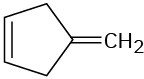
(b)
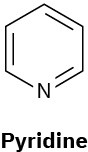
(c)
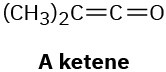
Problem 14-46
Would you expect allene, H2C=C=CH2, to show a UV absorption in the 200 to 400 nm range? Explain.
Problem 14-47
The following ultraviolet absorption maxima have been measured:
|
1,3-Butadiene |
217 nm |
|
2-Methyl-1,3-butadiene |
220 nm |
|
1,3-Pentadiene |
223 nm |
|
2,3-Dimethyl-1,3-butadiene |
226 nm |
|
2,4-Hexadiene |
227 nm |
|
2,4-Dimethyl-1,3-pentadiene |
232 nm |
|
2,5-Dimethyl-2,4-hexadiene |
240 nm |
What conclusion can you draw about the effect of alkyl substitution on UV absorption maxima? Approximately what effect does each added alkyl group have?
Problem 14-48
1,3,5-Hexatriene has λmax = 258 nm. In light of your answer to Problem 14-47, approximately where would you expect 2,3-dimethyl-1,3,5-hexatriene to absorb?
Problem 14-49
β-Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of β-ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments:
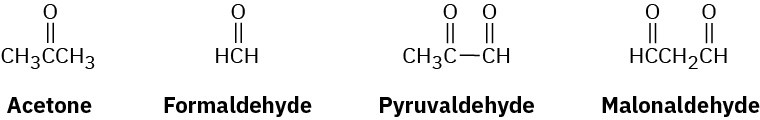
(a)
How many double bonds does β-ocimene have? (b)
Is β-ocimene conjugated or nonconjugated? (c)
Propose a structure for β-ocimene. (d)
Write the reactions, showing starting material and products.
General Problems
Problem 14-50
Draw the resonance forms that result when the following dienes are protonated. If the resonance forms differ in energy, identify the most stable one.
(a)
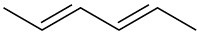
(b)
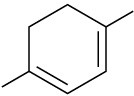
(c)
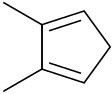
Problem 14-51
Answer the following questions for 1,3,5-cycloheptatriene. (a)
How many p atomic orbitals are in the conjugated system? (b)
How many molecular orbitals describe the conjugated system? (c)
How many molecular orbitals are bonding molecular orbitals? (d)
How many molecular orbitals are anti-bonding molecular orbitals? (e)
Which molecular orbitals are filled with electrons? (f)
If this molecule were to absorb a photon of UV light an electron would move between which two molecular orbitals (be specific)?
Problem 14-52
Treatment of 3,4-dibromohexane with strong base leads to loss of 2 equivalents of HBr and formation of a product with formula C6H10. Three products are possible. Name each of the three, and tell how you would use 1H and 13C NMR spectroscopy to help identify them. How would you use UV spectroscopy?
Problem 14-53
Addition of HCl to 1-methoxycyclohexene yields 1-chloro-1-methoxycyclohexane as a sole product. Use resonance structures to explain why none of the other regioisomer is formed.
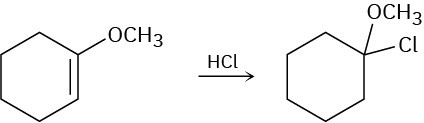
Problem 14-54
Aldrin, a chlorinated insecticide now banned from use in most countries since 1990, can be made by Diels–Alder reaction of hexachloro-1,3-cyclopentadiene with norbornadiene.
What is the structure of aldrin?
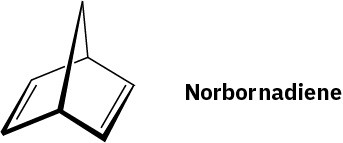
Problem 14-55
Norbornadiene (Problem 14-54) can be prepared by reaction of chloroethylene with 1,3- cyclopentadiene, followed by treatment of the product with sodium ethoxide. Write the overall scheme, and identify the two kinds of reactions.
Problem 14-56
The triene shown here reacts with 2 equivalents of maleic anhydride to yield a product with the formula C17H16O6. Predict a structure for the product.
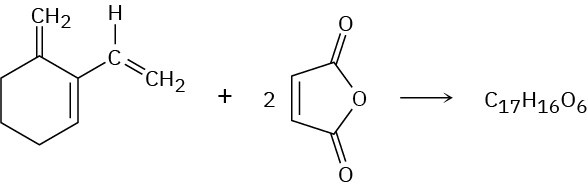
Problem 14-57
Myrcene, C10H16, is found in oil of bay leaves and is isomeric with β-ocimene (Problem 14- 49). It has an ultraviolet absorption at 226 nm and can be hydrogenated to yield 2,6- dimethyloctane. On ozonolysis followed by zinc/acetic acid treatment, myrcene yields formaldehyde, acetone, and 2-oxopentanedial:
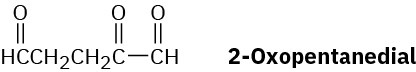
Propose a structure for myrcene, and write the reactions, showing starting material and products.
Problem 14-58
Hydrocarbon A, C10H14, has a UV absorption at λmax = 236 nm and gives hydrocarbon B, C10H18, on hydrogenation. Ozonolysis of A, followed by zinc/acetic acid treatment, yields the following diketo dialdehyde:
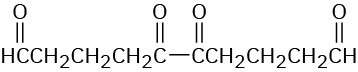
An illustration shows the structure of diketo dialdehyde. It shows two carbonyl groups single bonded to each other. Each carbonyl group is bonded to a chain of three methylene groups and then to an aldehyde group.
(a)
Propose two possible structures for A.
(b)
Hydrocarbon A reacts with maleic anhydride to yield a Diels–Alder adduct. Which of your structures for A is correct?
(c)
Write the reactions, showing the starting material and products. Problem 14-59
Adiponitrile, a starting material used in the manufacture of nylon, can be prepared in three steps from 1,3-butadiene. How would you carry out this synthesis?
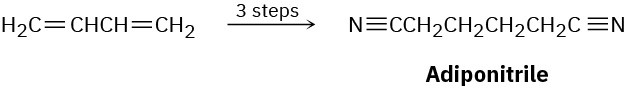
Problem 14-60
Ergosterol, a precursor of vitamin D, has λmax = 282 nm and molar absorptivity ϵ = 11,900. What is the concentration of ergosterol in a solution whose absorbance A = 0.065 with a sample pathlength l = 1.00 cm?
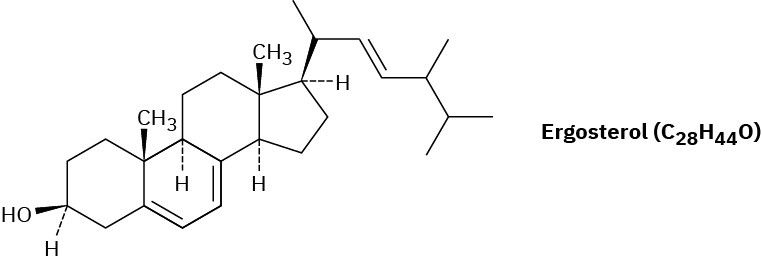
Problem 14-61
Dimethyl butynedioate undergoes a Diels–Alder reaction with (2E,4E)-2,4-hexadiene. Show the structure and stereochemistry of the product.
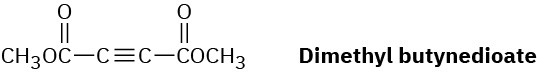
Problem 14-62
Dimethyl butynedioate also undergoes a Diels–Alder reaction with (2E,4Z)-2,4-hexadiene, but the stereochemistry of the product is different from that of the (2E,4E) isomer (Problem 14-61). Explain.
Problem 14-63
How would you carry out the following synthesis (more than one step is required)? What stereochemical relationship between the –CO2CH3 group attached to the cyclohexane ring and the –CHO groups would your synthesis produce?
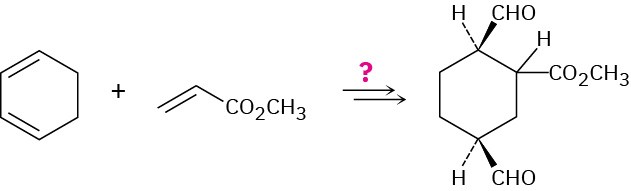
Problem 14-64
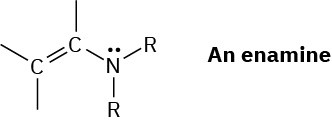
The double bond of an enamine (alkene + amine) is much more nucleophilic than a typical alkene double bond. Assuming that the nitrogen atom in an enamine is sp2-hybridized, draw an orbital picture of an enamine, and explain why the double bond is electron-rich.
Problem 14-65
Benzene has an ultraviolet absorption at λmax = 204 nm, and para-toluidine has λmax = 235 nm. How do you account for this difference?