Summary
Carboxylic acid derivatives—compounds in which the –OH group of a carboxylic acid has been replaced by another substituent—are among the most widely occurring of all molecules and are involved in almost all biological pathways. In this chapter, we covered the chemistry necessary for understanding them and thus also necessary for understanding living organisms. Acid halides, acid anhydrides, esters, and amides are the most common such derivatives in the laboratory; thioesters and acyl phosphates are common in biological molecules.
The chemistry of carboxylic acid derivatives is dominated by the nucleophilic acyl substitution reaction. Mechanistically, these substitutions take place by addition of a nucleophile to the polar carbonyl group of the acid derivative to give a tetrahedral intermediate, followed by expulsion of a leaving group.
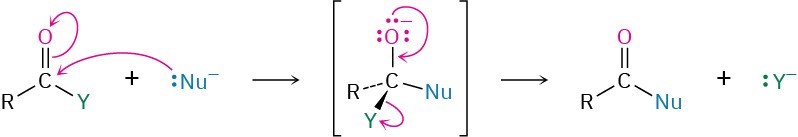
The reactivity of an acid derivative toward substitution depends both on the steric environment near the carbonyl group and on the electronic nature of the substituent, Y. The reactivity order is acid halide > acid anhydride > thioester > ester > amide.
The most common reactions of carboxylic acid derivatives are substitution by water to yield an acid (hydrolysis), by an alcohol to yield an ester (alcoholysis), by an amine to yield an amide (aminolysis), by hydride ion to yield an alcohol (reduction), and by an organomagnesium halide to yield an alcohol (Grignard reaction).
Step-growth polymers, such as polyamides and polyesters, are prepared by reactions between difunctional molecules. Polyamides (nylons) are formed by reaction between a diacid and a diamine; polyesters are formed from a diacid and a diol.
IR spectroscopy is a valuable tool for the structural analysis of acid derivatives. Acid chlorides, anhydrides, esters, and amides all show characteristic IR absorptions that can be used to identify these functional groups.

