15.3 Aromaticity and the Hückel 4n + 2 Rule
Let’s list what we’ve said thus far about benzene and, by extension, about other benzene- like aromatic molecules.
- Benzene is cyclic and conjugated.
- Benzene is unusually stable, having a heat of hydrogenation 150 kJ/mol less negative than we might expect for a conjugated cyclic triene.
- Benzene is planar and has the shape of a regular hexagon. All bond angles are 120°, all carbon atoms are sp2-hybridized, and all carbon–carbon bond lengths are 139 pm.
- Benzene undergoes substitution reactions that retain the cyclic conjugation rather than electrophilic addition reactions that would destroy it.
- Benzene can be described as a resonance hybrid whose structure is intermediate between two line-bond structures.
This list might seem to be a good description of benzene and other aromatic molecules, but it isn’t enough. Something else, called the Hückel 4n + 2 rule, is needed to complete a description of aromaticity. According to a theory devised in 1931 by the German physicist Erich Hückel, a molecule is aromatic only if it has a planar, monocyclic system of conjugation and contains a total of 4n + 2 π electrons, where n is an integer (n = 0, 1, 2, 3,). In other words, only molecules with 2, 6, 10, 14, 18, π electrons can be aromatic. Molecules with 4n π electrons (4, 8, 12, 16,) can’t be aromatic, even though they may be cyclic, planar, and apparently conjugated. In fact, planar, conjugated molecules with 4n π electrons are said to be antiaromatic because delocalization of their π electrons would lead to their destabilization.
Let’s look at several examples to see how the Hückel 4n + 2 rule works.
- Cyclobutadiene has four π electrons and is antiaromatic. As indicated by the electrostatic potential map, the π electrons are localized in two double bonds rather than delocalized around the ring.
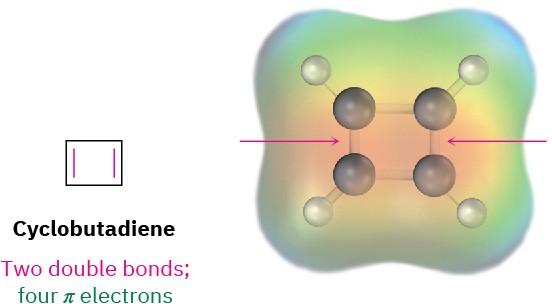
Cyclobutadiene is highly reactive and shows none of the properties associated with
aromaticity. In fact, it was not even prepared until 1965, when Rowland Pettit at the University of Texas was able to make it at low temperature. Even at –78 °C, however, cyclobutadiene is so reactive that it dimerizes by a Diels–Alder reaction.
One molecule behaves as a diene and the other as a dienophile.

- Benzene has six π electrons (4n + 2 = 6 when n = 1) and is aromatic.

- Cyclooctatetraene has eight π electrons, but when it was first prepared in 1911 by the German chemist Richard Willstätter, it was found not to be particularly stable. In fact, its π electrons are localized into four double bonds rather than delocalized around the ring, and the molecule is tub-shaped rather than planar. It has no cyclic conjugation because neighboring p orbitals don’t have the necessary parallel alignment for overlap, and it resembles an open-chain polyene in its reactivity.
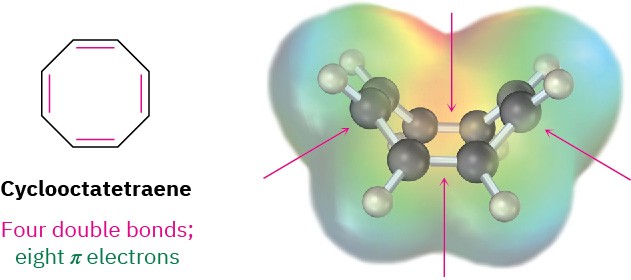
X-ray studies show that the C–C single bonds in cyclooctatetraene are 147 pm long and the double bonds are 134 pm long. In addition, the 1H NMR spectrum shows a single sharp resonance line at 5.78 δ, a value characteristic of an alkene rather than an aromatic molecule.
What’s so special about 4n + 2 π electrons? Why do 2, 6, 10, 14 π electrons lead to aromatic stability, while other numbers of electrons do not? The answer comes from molecular orbital theory. When the energy levels of molecular orbitals for cyclic conjugated molecules are calculated, it turns out that there is always a single lowest-lying MO, above which the MOs come in degenerate pairs. Thus, when electrons fill the various molecular orbitals, it takes two electrons, or one pair, to fill the lowest-lying orbital and four electrons, or two pairs, to fill each of n succeeding energy levels—a total of 4n + 2. Any other number would leave an energy level partially filled.
The six π molecular orbitals of benzene were shown previously in Figure 15.5, and their relative energies are shown again in Figure 15.6. The lowest-energy MO, ψ1, occurs singly and contains two electrons. The next two lowest-energy orbitals, ψ2 and ψ3, are degenerate,
and it therefore takes four electrons to fill both. The result is a stable six-π-electron aromatic molecule with filled bonding orbitals.
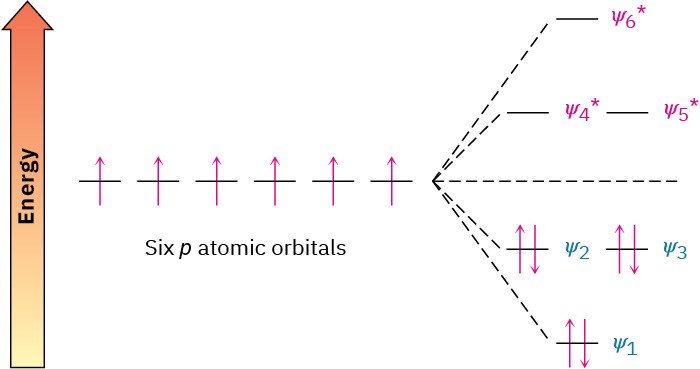
Figure 15.6 Energy levels of the six benzene π molecular orbitals. There is a single, lowest-energy orbital, above which the orbitals come in degenerate pairs.
Problem 15-5
To be aromatic, a molecule must have 4n + 2 π electrons and must have a planar, monocyclic system of conjugation. Cyclodecapentaene fulfills one of these criteria but not the other and has resisted all attempts at synthesis. Explain.