11.9 The E2 Reaction and Cyclohexane Conformation
Anti periplanar geometry for E2 reactions is particularly important in cyclohexane rings, where chair geometry forces a rigid relationship between the substituents on neighboring carbon atoms (Section 4.8). The anti periplanar requirement for E2 reactions overrides Zaitsev’s rule and can be met in cyclohexanes only if the hydrogen and the leaving group are trans diaxial (Figure 11.20). If either the leaving group or the hydrogen is equatorial, E2 elimination can’t occur.
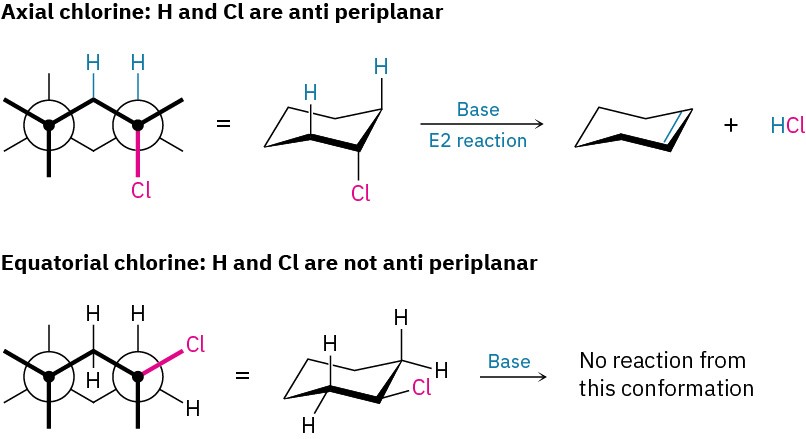
Figure 11.20 The geometric requirement for an E2 reaction in a substituted cyclohexane. The leaving group and the hydrogen must both be axial for anti periplanar elimination to occur.
The elimination of HCl from the isomeric menthyl and neomenthyl chlorides shown in Figure 11.21 gives a good illustration of this trans-diaxial requirement. Neomenthyl chloride undergoes elimination of HCl on reaction with ethoxide ion 200 times faster than menthyl chloride. Furthermore, neomenthyl chloride yields 3-menthene as the major alkene product, whereas menthyl chloride yields 2-menthene.
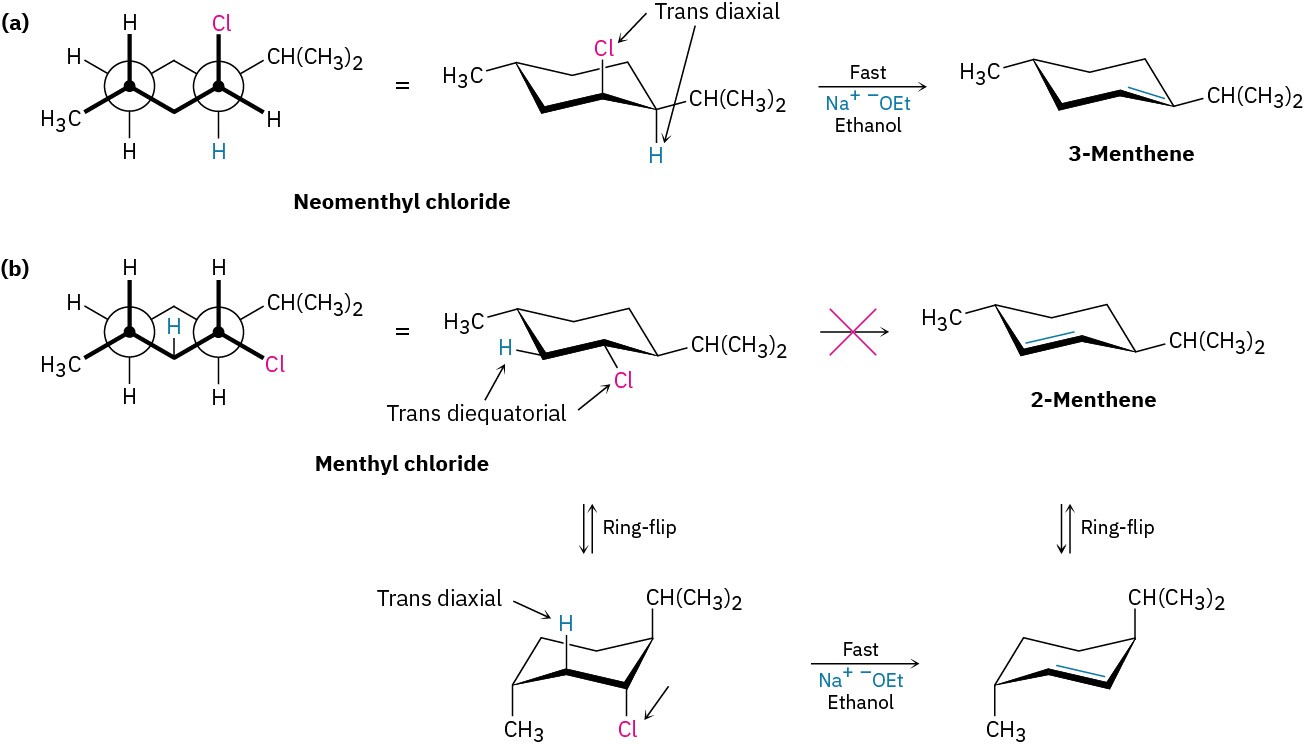
Figure 11.21 Dehydrochlorination of menthyl and neomenthyl chlorides. (a) Neomenthyl chloride loses HCl directly from its more stable conformation, but (b) menthyl chloride must first ring-flip to a higher energy conformation before HCl loss can occur. The abbreviation “Et” represents an ethyl group.
The difference in reactivity between the isomeric menthyl chlorides is due to the difference in their conformations. Neomenthyl chloride has the conformation shown in Figure 11.21a, with the methyl and isopropyl groups equatorial and the chlorine axial—a perfect geometry for E2 elimination. Loss of the hydrogen atom at C4 occurs easily to yield the more substituted alkene product, 3-menthene, as predicted by Zaitsev’s rule.
Menthyl chloride, by contrast, has a conformation in which all three substituents are equatorial (Figure 11.21b). To achieve the necessary geometry for elimination, menthyl chloride must first ring-flip to a higher-energy chair conformation, in which all three substituents are axial. E2 elimination then occurs with loss of the only trans-diaxial hydrogen available, leading to the non-Zaitsev product 2-menthene. The net effect of the simple change in chlorine stereochemistry is a 200-fold change in reaction rate and a complete change of product. The chemistry of the molecule is controlled by its conformation.
Problem 11-19
Which isomer would you expect to undergo E2 elimination faster, trans-1-bromo-4-tert– butylcyclohexane or cis-1-bromo-4-tert-butylcyclohexane? Draw each molecule in its more stable chair conformation, and explain your answer.

