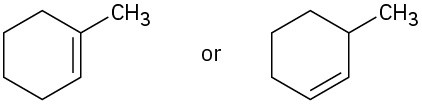7.6 Stability of Alkenes
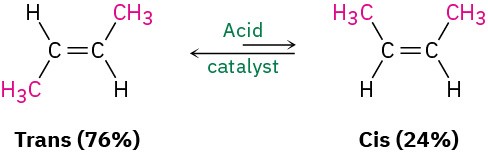
Although the cis–trans interconversion of alkene isomers does not occur spontaneously, it can often be made to happen by treating the alkene with a strong acid catalyst. If we interconvert cis-2-butene with trans-2-butene and allow them to reach equilibrium, we find that they aren’t of equal stability. The trans isomer is more stable than the cis isomer by 2.8 kJ/mol (0.66 kcal/mol) at room temperature, corresponding to a 76 : 24 ratio.
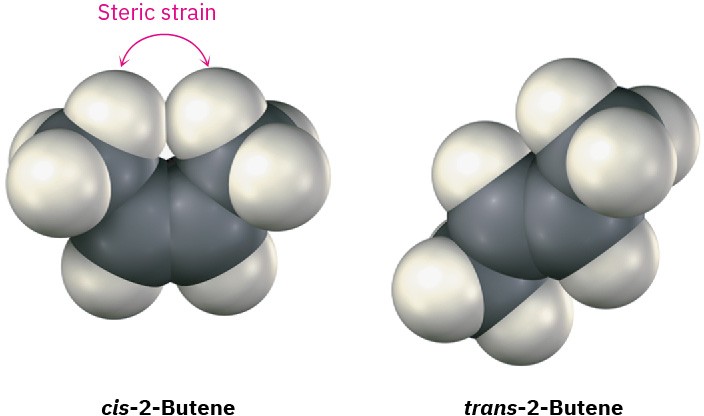
Cis alkenes are less stable than their trans isomers because of steric strain between the two larger substituents on the same side of the double bond. This is the same kind of steric interference that we saw previously in the axial conformation of methylcyclohexane (Section 4.7).
Although it’s sometimes possible to find relative stabilities of alkene isomers by establishing a cis–trans equilibrium through treatment with strong acid, a more general method is to take advantage of the fact that alkenes undergo a hydrogenation reaction to give the corresponding alkane when treated with H2 gas in the presence of a catalyst such as palladium or platinum.
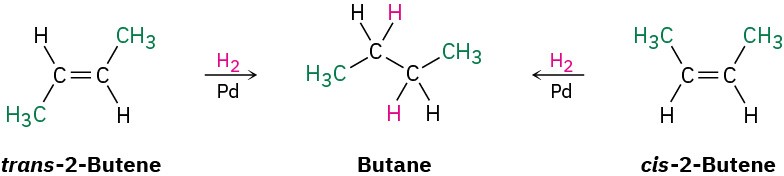
Energy diagrams for the hydrogenation reactions of cis– and trans-2-butene are shown in Figure 7.6. Because cis-2-butene is less stable than trans-2-butene by 2.8 kJ/mol, the energy diagram shows the cis alkene at a higher energy level. After reaction, however, both curves
are at the same energy level (butane). It therefore follows that ΔG° for reaction of the cis isomer must be larger than ΔG° for reaction of the trans isomer by 2.8 kJ/mol. In other words, more energy is released in the hydrogenation of the cis isomer than the trans isomer because the cis isomer has more energy to begin with.
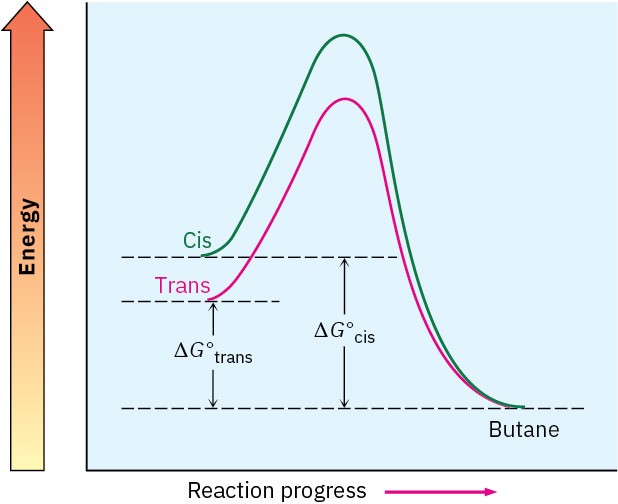
Figure 7.6 Energy diagrams for hydrogenation of cis– and trans-2-butene. The cis isomer is higher in energy than the trans isomer by about 2.8 kJ/mol and therefore releases more energy when hydrogenated.
If we were to measure the so-called heats of hydrogenation (ΔH°hydrog) for two double-bond isomers and find their difference, we could determine the relative stabilities of cis and trans isomers without having to measure an equilibrium position. cis-2-Butene, for instance, has ΔH°hydrog = −119 kJ/mol(−28.3 kcal/mol), while trans-2-butene has ΔH°hydrog =
−115 kJ/mol(−27.4 kcal/mol)—a difference of 4 kJ/mol.
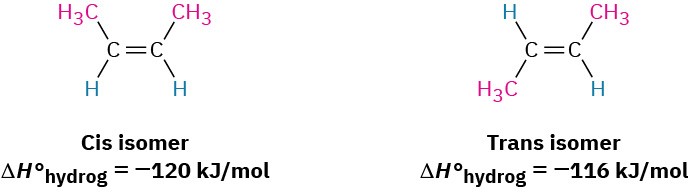
The 4 kJ/mol energy difference between the 2-butene isomers calculated from heats of hydrogenation agrees reasonably well with the 2.8 kJ/mol energy difference calculated from equilibrium data, but the values aren’t exactly the same for two reasons. First, there is probably some experimental error, because heats of hydrogenation are difficult to measure accurately. Second, heats of reaction and equilibrium constants don’t measure exactly the same thing. Heats of reaction measure enthalpy changes, ΔH°, whereas equilibrium constants measure free-energy changes, ΔG°, so we might expect a slight difference between the two.
Table 7.2 Heats of Hydrogenation of Some Alkenes
|
|
|
(kJ/mol) |
(kcal/mol) |
|
Ethylene |
H!C═CH! |
−136 |
−32.6 |
|
Monosubstituted |
CH“CH═CH! |
−125 |
−29.9 |
|
Disubstituted |
CH“CH═CHCH“ (cis) |
−119 |
−28.3 |
|
|
CH“CH═CHCH“ (trans) |
−115 |
−27.4 |
|
|
(CH”)!C═CH! |
−118 |
−28.2 |
|
Trisubstituted |
(CH”)!C═CHCH“ |
−112 |
−26.7 |
|
Tetrasubstituted |
(CH”)!C═C(CH”)! |
−110 |
−26.4 |
Table 7.2 lists some representative data for the hydrogenation of different alkenes and shows that alkenes become more stable with increasing substitution. That is, alkenes follow the stability order:
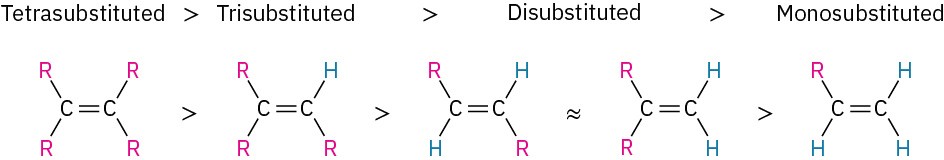
The stability order of substituted alkenes is due to a combination of two factors. One is a stabilizing interaction between the C═C π orbital and adjacent C−H σ bonds on substituents. In valence-bond language, the interaction is called hyperconjugation. In a molecular orbital description, there is a bonding MO that extends over the four-atom C═C−C−H grouping, as shown in Figure 7.7. The more substituents present on the double bond, the more hyperconjugation occurs and the more stable the alkene.
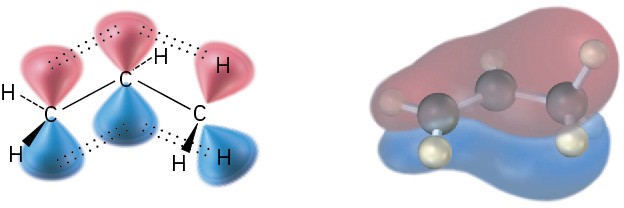
Figure 7.7 Hyperconjugation is a stabilizing interaction between the C═C π orbital and adjacent C−H σ bonds on substituents. The more substituents there are, the greater the stabilization of the alkene.
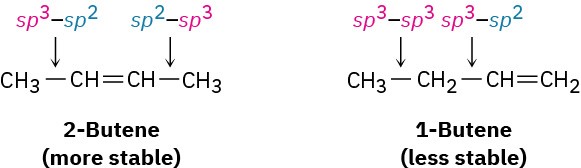
A second factor that contributes to alkene stability involves bond strengths. A bond between a sp2 carbon and a sp3 carbon is somewhat stronger than a bond between two sp3 carbons. Thus, in comparing 1-butene and 2-butene, the monosubstituted isomer has one sp3–sp3 bond and one sp3–sp2 bond, while the disubstituted isomer has two sp3–sp2 bonds. More highly substituted alkenes always have a higher ratio of sp3–sp2 bonds to sp3–sp3 bonds than less highly substituted alkenes and are therefore more stable.
Problem 7-15
Name the following alkenes, and tell which compound in each pair is more stable: (a)
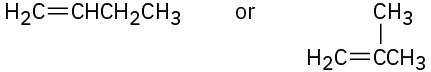
(b)
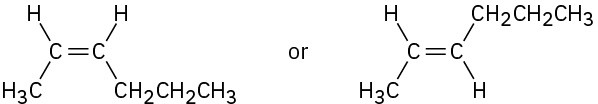
(c)