4.9 Conformations of Polycyclic Molecules
The final point we’ll consider about cycloalkane stereochemistry is to see what happens when two or more cycloalkane rings are fused together along a common bond to construct a polycyclic molecule—for example, decalin.

Decalin consists of two cyclohexane rings joined to share two carbon atoms (the bridgehead carbons, C1 and C6) and a common bond. Decalin can exist in either of two isomeric forms, depending on whether the rings are trans fused or cis fused. In cis-decalin, the hydrogen atoms at the bridgehead carbons are on the same side of the rings; in trans– decalin, the bridgehead hydrogens are on opposite sides. Figure 4.18 shows how both compounds can be represented using chair cyclohexane conformations. Note that the two decalin isomers are not interconvertible by ring-flips or other rotations. They are cis–trans stereoisomers and have the same relationship to each other that cis– and trans-1,2- dimethylcyclohexane have.
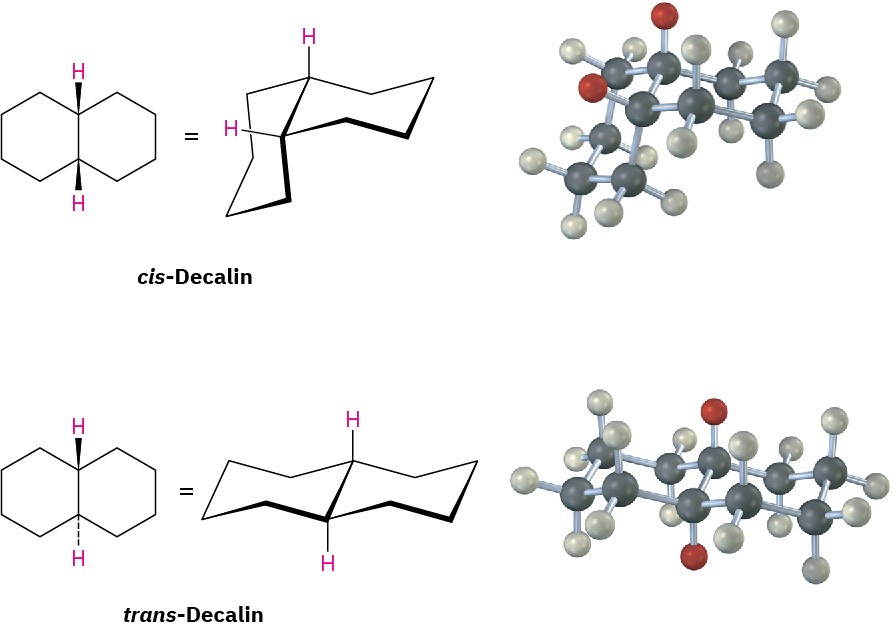
Figure 4.18 Representations of cis– and trans-decalin. Hydrogen atoms at the bridgehead carbons are on the same face of the rings in the cis isomer but on opposite faces in the trans isomer.
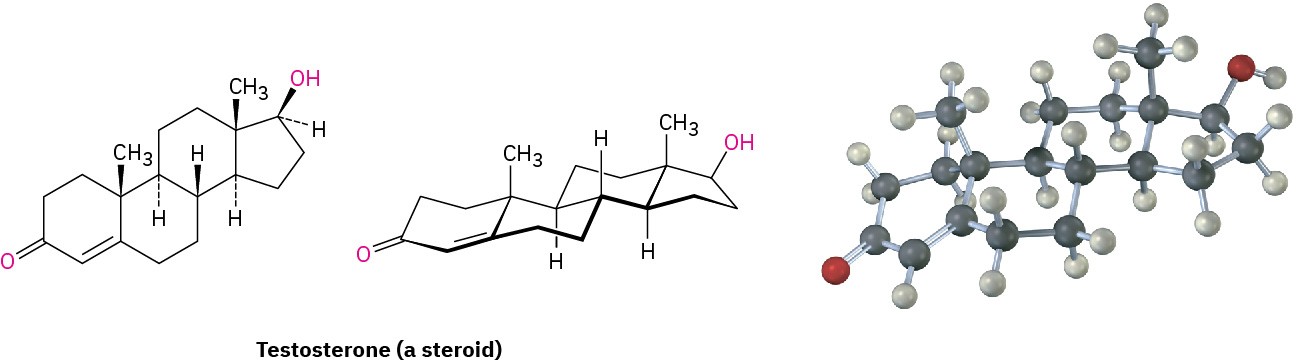
Polycyclic compounds are common in nature, and many valuable substances have fused- ring structures. For example, steroids, such as testosterone, the primary sex hormone in
males, have three six-membered rings and one five-membered ring fused together. Although steroids look complicated compared with cyclohexane or decalin, the same principles that apply to the conformational analysis of simple cyclohexane rings apply equally well (and often better) to steroids.
Another common ring system is the norbornane, or bicyclo[2.2.1]heptane, structure. Like decalin, norbornane is a bicycloalkane, so called because two rings would have to be broken open to generate an acyclic structure. Its systematic name, bicyclo[2.2.1]heptane, reflects the fact that the molecule has seven carbons, is bicyclic, and has three “bridges” of 2, 2, and 1 carbon atoms connecting the two bridgehead carbons.
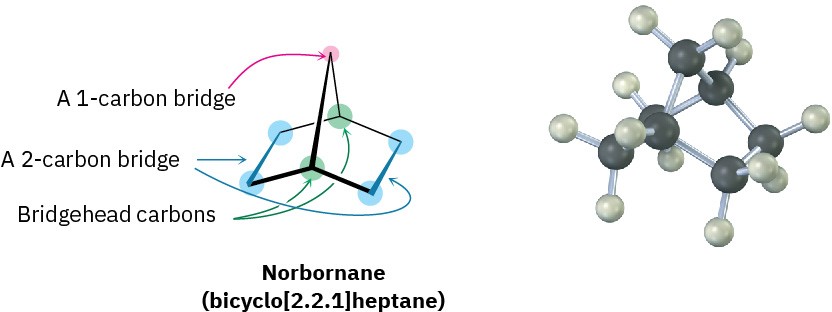
Norbornane has a conformationally locked boat cyclohexane ring (Section 4.5) in which carbons 1 and 4 are joined by an additional CH2 group. In drawing this structure, a break in the rear bond indicates that the vertical bond crosses in front of it. Making a molecular model is particularly helpful when trying to see the three-dimensionality of norbornane.
Substituted norbornanes, such as camphor, are found widely in nature, and many have been important historically in developing organic structural theories.
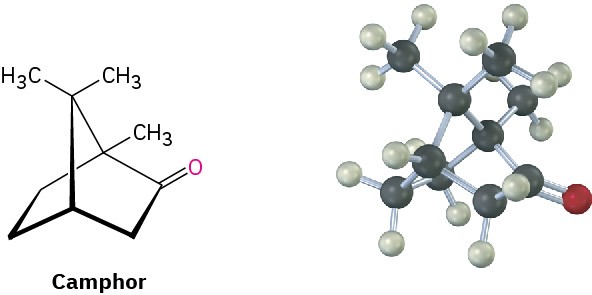
Problem 4-20
Which isomer is more stable, cis-decalin or trans-decalin (Figure 4.18)? Explain. Problem 4-21
Look at the following structure of estrone, the primary sex hormone in females, and tell whether each of the two indicated (red) ring-fusions is cis or trans.
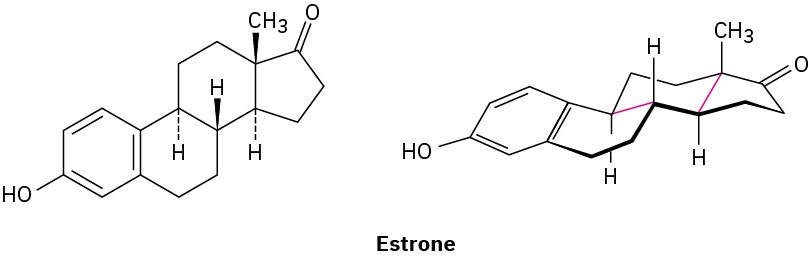
Additional Problems 4 • Additional Problems 4 • Additional Problems Visualizing Chemistry Problem 4-22
Name the following cycloalkanes:
(a)
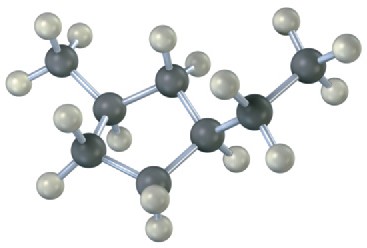
(b)
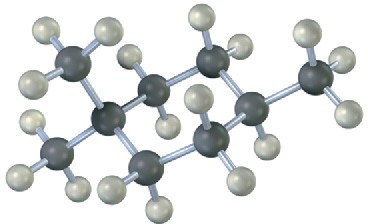
Problem 4-23
Name the following compound, identify each substituent as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):
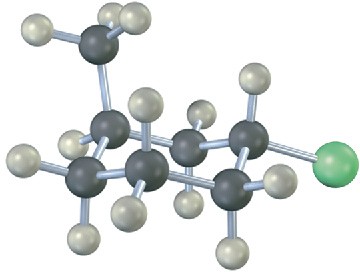
Problem 4-24
A trisubstituted cyclohexane with three substituents—red, green, and blue—undergoes a ring-flip to its alternate chair conformation. Identify each substituent as axial or equatorial, and show the positions occupied by the three substituents in the ring-flipped form.
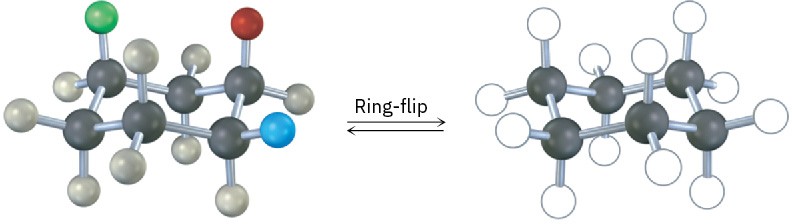
Problem 4-25
The following cyclohexane derivative has three substituents—red, green, and blue. Identify each substituent as axial or equatorial, and identify each pair of relationships (red–blue, red–green, and blue–green) as cis or trans.
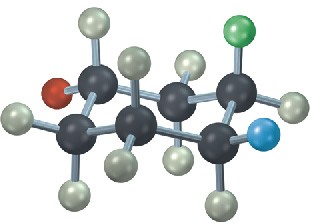
Problem 4-26
Glucose exists in two forms having a 36:64 ratio at equilibrium. Draw a skeletal structure of each, describe the difference between them, and tell which of the two you think is more stable (red = O).
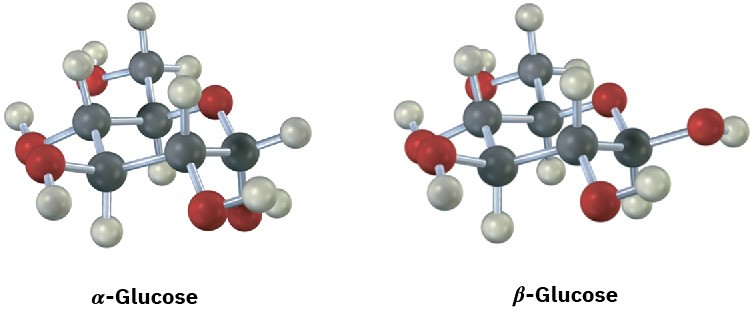
Cycloalkane Isomers
Problem 4-27
Draw the five cycloalkanes with the formula C5H10. Problem 4-28
Give IUPAC names for the following compounds. (a)
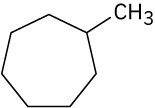
(b)
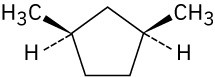
(c)
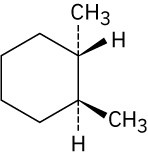
(d)
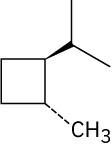
(e)
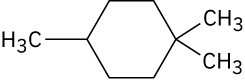
Problem 4-29
Draw a stereoisomer of trans-1,3-dimethylcyclobutane. Problem 4-30
Tell whether the following pairs of compounds are identical, constitutional isomers, stereoisomers, or unrelated.
(a)
cis-1,3-Dibromocyclohexane and trans-1,4-dibromocyclohexane (b)
2,3-Dimethylhexane and 2,3,3-trimethylpentane (c)

Problem 4-31
Draw three isomers of trans-1,2-dichlorocyclobutane, and label them as either constitutional isomers or stereoisomers.
Problem 4-32
Identify each pair of relationships among the –OH groups in glucose (red–blue, red–green, red–black, blue–green, blue–black, green–black) as cis or trans.
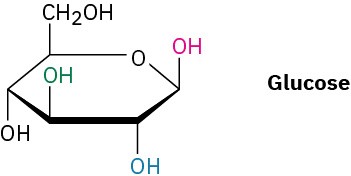
Problem 4-33
Draw 1,3,5-trimethylcyclohexane using a hexagon to represent the ring. How many cis– trans stereoisomers are possible?
Cycloalkane Conformation and Stability
Problem 4-34
Hydrocortisone, a naturally occurring hormone produced in the adrenal glands, is often used to treat inflammation, severe allergies, and numerous other conditions. Is the indicated −OH group axial or equatorial?
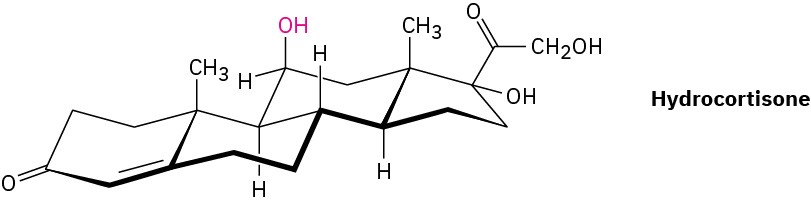
Problem 4-35
A 1,2-cis disubstituted cyclohexane, such as cis-1,2-dichlorocyclohexane, must have one group axial and one group equatorial. Explain.
Problem 4-36
A 1,2-trans disubstituted cyclohexane must have either both groups axial or both groups equatorial. Explain.
Problem 4-37
Why is a 1,3-cis disubstituted cyclohexane more stable than its trans isomer? Problem 4-38
Which is more stable, a 1,4-trans disubstituted cyclohexane or its cis isomer?
Problem 4-39
cis-1,2-Dimethylcyclobutane is less stable than its trans isomer, but cis-1,3- dimethylcyclobutane is more stable than its trans isomer. Draw the most stable conformations of both, and explain.
Problem 4-40
From the data in Figure 4.13 and Table 4.1, estimate the percentages of molecules that have their substituents in an axial orientation for the following compounds:
(a) Isopropylcyclohexane (b) Fluorocyclohexane (c)
Cyclohexanecarbonitrile, C6H11CN Problem 4-41
Assume that you have a variety of cyclohexanes substituted in the positions indicated. Identify the substituents as either axial or equatorial. For example, a 1,2-cis relationship means that one substituent must be axial and one equatorial, whereas a 1,2-trans relationship means that both substituents are axial or both are equatorial.
(a)
1,3-Trans disubstituted (b)
1,4-Cis disubstituted (c)
1,3-Cis disubstituted (d)
1,5-Trans disubstituted (e)
1,5-Cis disubstituted (f)
1,6-Trans disubstituted
Cyclohexane Conformational Analysis
Problem 4-42
Draw the two chair conformations of cis-1-chloro-2-methylcyclohexane. Which is more stable, and by how much?
Problem 4-43
Draw the two chair conformations of trans-1-chloro-2-methylcyclohexane. Which is more stable?
Problem 4-44
Galactose, a sugar related to glucose, contains a six-membered ring in which all the substituents except the –OH group, indicated below in red, are equatorial. Draw galactose in its more stable chair conformation.
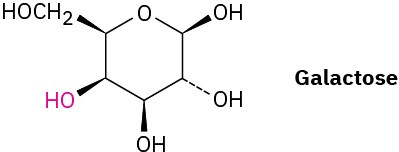
Problem 4-45
Draw the two chair conformations of menthol, and tell which is more stable.
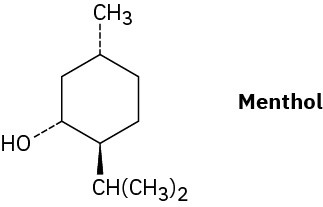
Problem 4-46
There are four cis–trans isomers of menthol (Problem 4-45), including the one shown. Draw the other three.
Problem 4-47
The diaxial conformation of cis-1,3-dimethylcyclohexane is approximately 23 kJ/mol (5.4 kcal/mol) less stable than the diequatorial conformation. Draw the two possible chair conformations, and suggest a reason for the large energy difference.
Problem 4-48
Approximately how much steric strain does the 1,3-diaxial interaction between the two methyl groups introduce into the diaxial conformation of cis-1,3-dimethylcyclohexane? (See Problem 4-47.)
Problem 4-49
In light of your answer to Problem 4-48, draw the two chair conformations of 1,1,3- trimethylcyclohexane and estimate the amount of strain energy in each. Which conformation is favored?
Problem 4-50
One of the two chair structures of cis-1-chloro-3-methylcyclohexane is more stable than the other by 15.5 kJ/mol (3.7 kcal/mol). Which is it? What is the energy cost of a 1,3-diaxial interaction between a chlorine and a methyl group?
General Problems
Problem 4-51
We saw in Problem 4-20 that cis-decalin is less stable than trans-decalin. Assume that the 1,3-diaxial interactions in cis-decalin are similar to those in axial methylcyclohexane [that is, one CH2↔H interaction costs 3.8 kJ/mol (0.9 kcal/mol)], and calculate the magnitude of the energy difference between cis– and trans-decalin.
Problem 4-52
Using molecular models as well as structural drawings, explain why trans-decalin is rigid and cannot ring-flip whereas cis-decalin can easily ring-flip.
Problem 4-53
trans-Decalin is more stable than its cis isomer, but cis-bicyclo[4.1.0]heptane is more stable than its trans isomer. Explain.
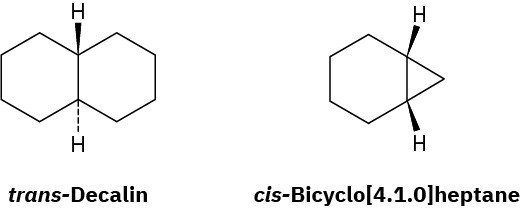
Problem 4-54
As mentioned in Problem 3-53, the statin drugs, such as simvastatin (Zocor), pravastatin (Pravachol), and atorvastatin (Lipitor) are the most widely prescribed drugs in the world.
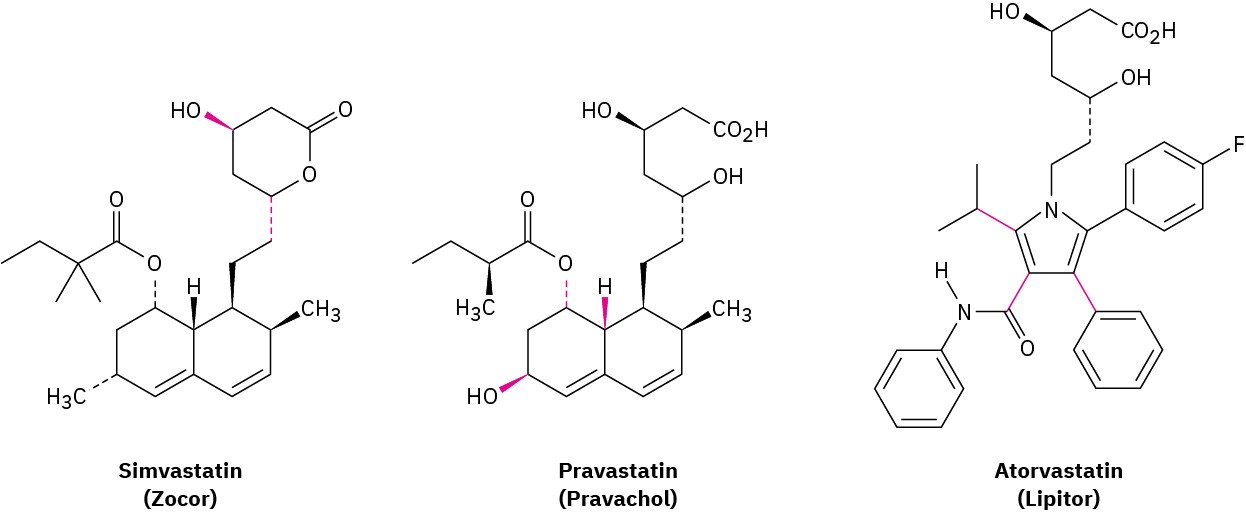
(a)
Are the two indicated bonds on simvastatin cis or trans? (b)
What are the cis/trans relationships among the three indicated bonds on pravastatin? (c)
Why can’t the three indicated bonds on atorvastatin be identified as cis or trans?
Problem 4-55
myo-Inositol, one of the isomers of 1,2,3,4,5,6-hexahydroxycyclohexane, acts as a growth factor in both animals and microorganisms. Draw the most stable chair conformation of myo-inositol.
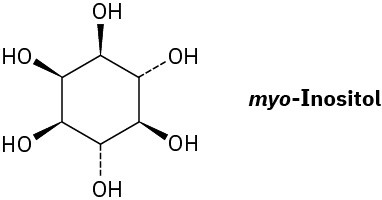
Problem 4-56
How many cis–trans stereoisomers of myo-inositol (Problem 4-55) are there? Draw the structure of the most stable isomer.
Problem 4-57
Julius Bredt, discoverer of the structure of camphor, proposed in 1935 that bicycloalkenes such as 1-norbornene, which have a double bond to a bridgehead carbon, are too strained to exist. Explain. (Making a molecular model will be helpful.
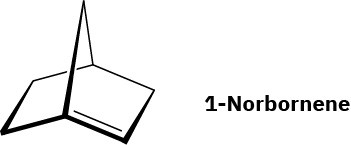
Problem 4-58
Tell whether each of the following substituents on a steroid is axial or equatorial. (A substituent that is “up” is on the top side of the molecule as drawn, and a substituent that is “down” is on the bottom side.
(a)
Substituent up at C3 (b)
Substituent down at C7 (c)
Substituent down at C11
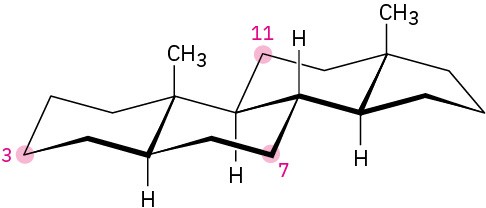
Problem 4-59
Amantadine is an antiviral agent that is active against influenza type A infection. Draw a three-dimensional representation of amantadine, showing the chair cyclohexane rings.
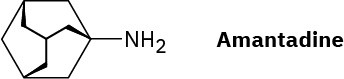
Problem 4-60
There are two different isomers named trans-1,2-dimethylcyclopentane. Similarly, you have two different appendages called hands. What is the relationship between them? (We’ll explore this kind of isomerism in the next chapter.)

Problem 4-61
Ketones react with alcohols to yield products called ketals. Why does the all-cis isomer of 4- tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form a ketal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product ketal for each one.
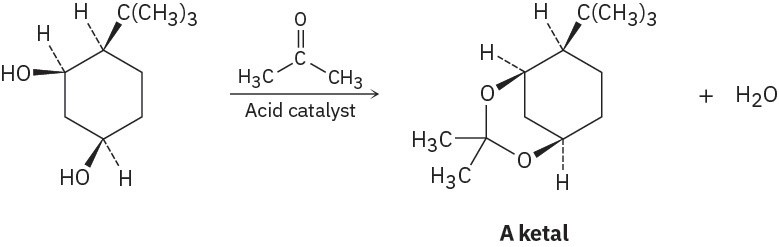
Problem 4-62
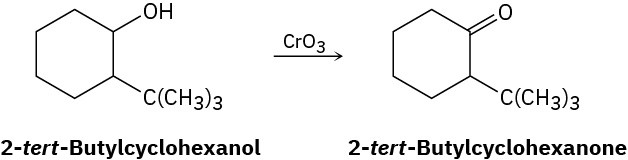
Alcohols undergo an oxidation reaction to yield carbonyl compounds when treatment with CrO3. For example, 2-tert-butylcyclohexanol gives 2-tert-butylcyclohexanone. If axial −OH groups are generally more reactive than their equatorial isomers, which do you think reacts faster, the cis isomer of 2-tert-butylcyclohexanol or the trans isomer? Explain.