27.7 Biosynthesis of Steroids
Steroids are heavily modified triterpenoids that are biosynthesized in living organisms from farnesyl diphosphate (C15). A reductive dimerization first converts farnesyl diphosphate to the acyclic hydrocarbon squalene (C30), which is converted into lanosterol (Figure 27.13). Further rearrangements and degradations then take place to yield various steroids. The conversion of squalene to lanosterol is among the most intensively studied of biosynthetic transformations. Starting from an achiral, open-chain polyene, the entire process requires only two enzymes and results in the formation of six carbon–carbon bonds, four rings, and seven chirality centers.
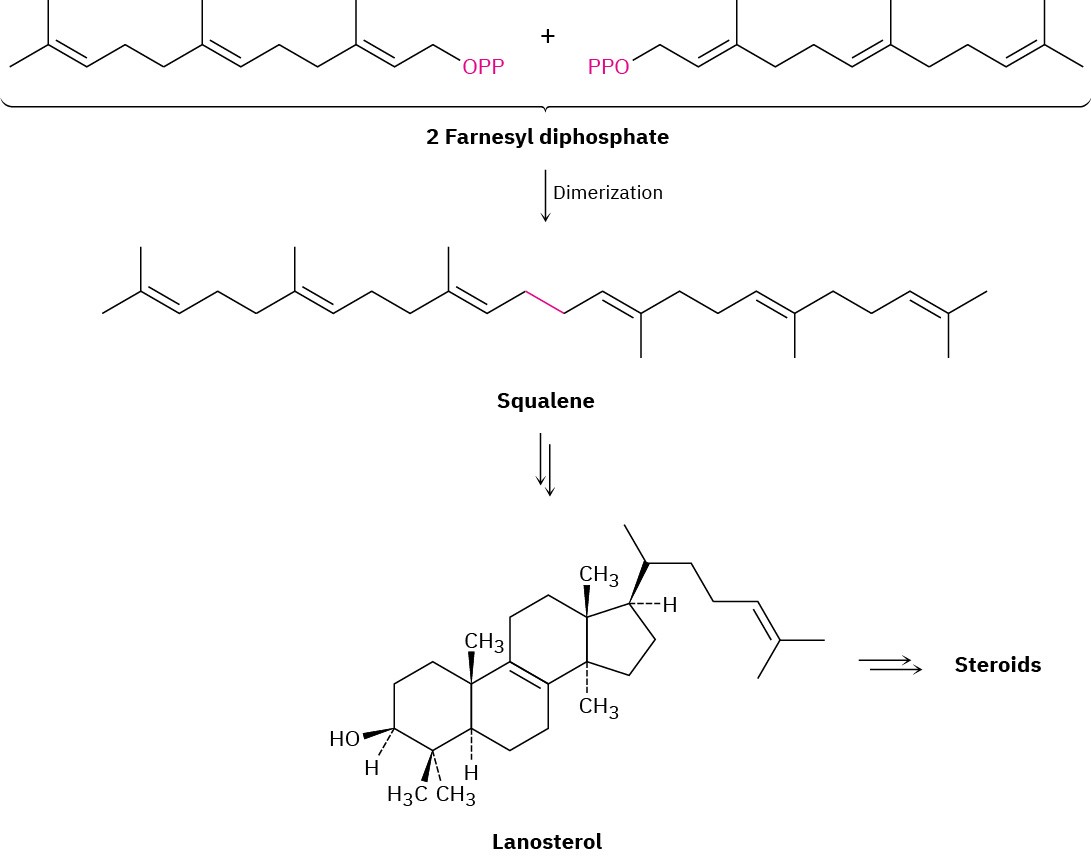
Figure 27.13An overview of steroid biosynthesis from farnesyl diphosphate.
Lanosterol biosynthesis begins with the selective epoxidation of squalene to give (3S)-2,3- oxidosqualene, catalyzed by squalene epoxidase. Molecular O2 provides the epoxide oxygen atom, and NADPH is required, along with a flavin coenzyme. The proposed mechanism involves reaction of FADH2 with O2 to produce a flavin hydroperoxide intermediate (ROOH), which transfers an oxygen to squalene in a pathway initiated by nucleophilic attack of the squalene double bond on the terminal hydroperoxide oxygen (Figure 27.14). The flavin alcohol formed as a by-product loses H2O to give FAD, which is reduced back to FADH2 by NADPH. As noted in Section 8.7, this biological epoxidation mechanism is closely
analogous to the mechanism by which peroxyacids (RCO3H) react with alkenes to give epoxides in the laboratory.
The second part of lanosterol biosynthesis is catalyzed by oxidosqualene-lanosterol cyclase and occurs as shown in Figure 27.15. Squalene is folded by the enzyme into a conformation that aligns the various double bonds for a cascade of successive intramolecular electrophilic additions, followed by a series of hydride and methyl migrations. Except for the initial epoxide protonation/cyclization, the process is probably stepwise and appears to involve discrete carbocation intermediates that are stabilized by electrostatic interactions with electron-rich aromatic amino acids in the enzyme.
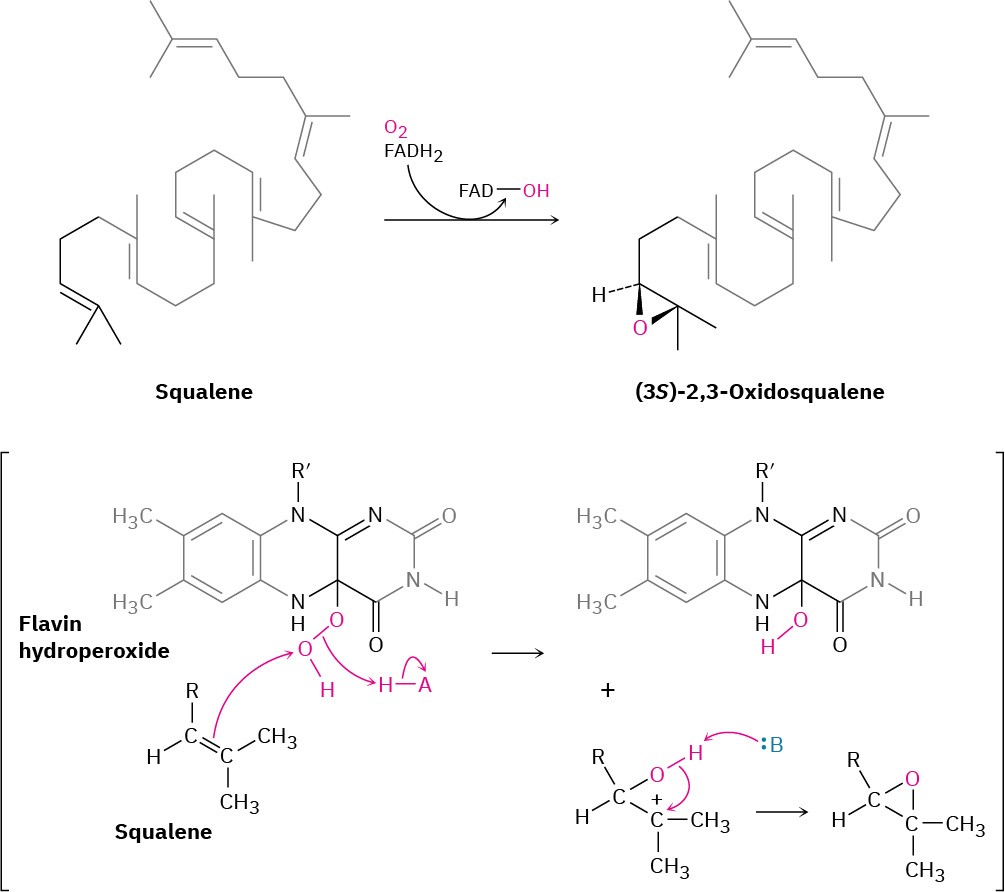
Figure 27.14Proposed mechanism of the oxidation of squalene by flavin hydroperoxide.
Steps 1, 2 of Figure 27.15: Epoxide Opening and Initial Cyclizations
Cyclization begins in step 1 with protonation of the epoxide ring by an aspartic acid residue in the enzyme. Nucleophilic opening of the protonated epoxide by the nearby 5,10 double bond (steroid numbering; Section 27.6) then yields a tertiary carbocation at C10. Further addition of C10 to the 8,9 double bond in step 2 next gives a bicyclic tertiary cation at C8.
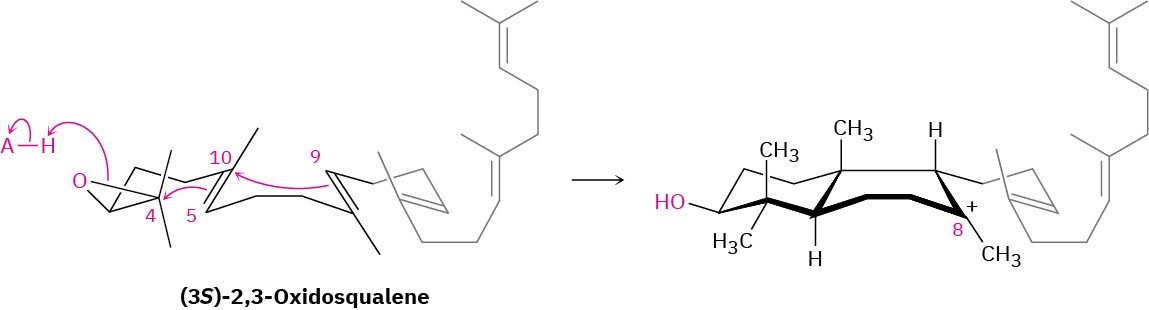
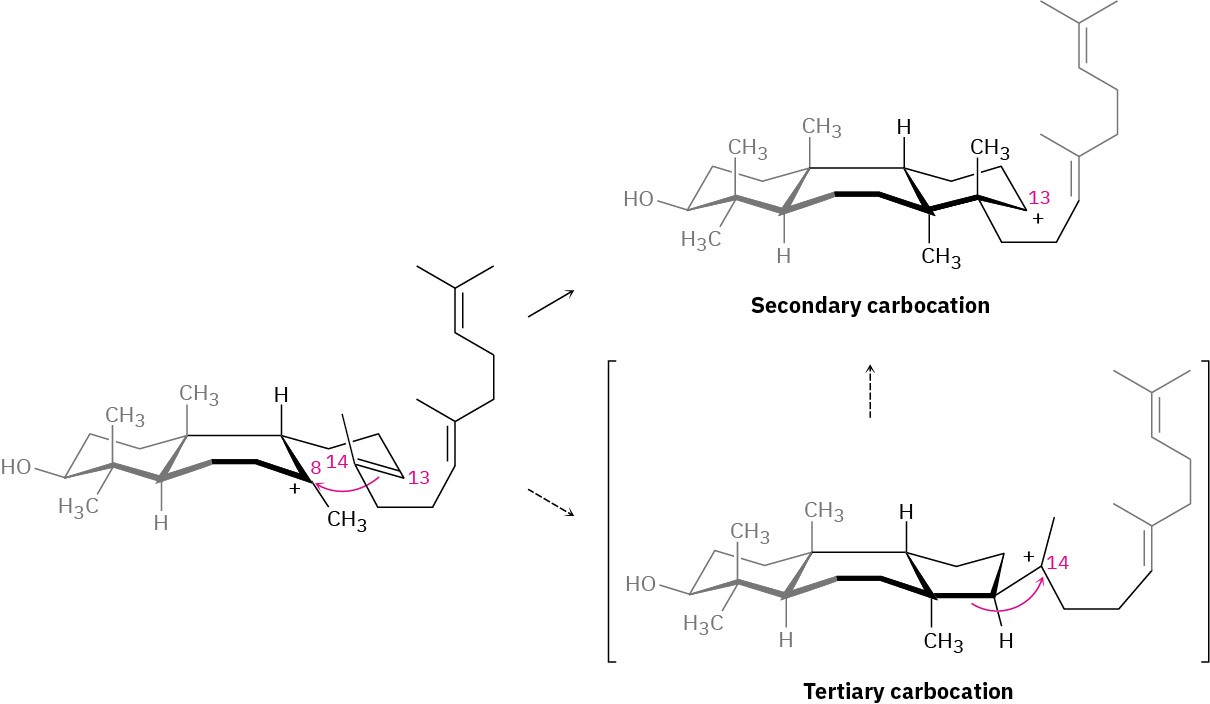
Step 3 of Figure 27.15: Third Cyclization
The third cationic cyclization is somewhat unusual because it occurs with non- Markovnikov regiochemistry and gives a secondary cation at C13 rather than the alternative tertiary cation at C14. There is growing evidence, however, that the tertiary carbocation may in fact be formed initially and that the secondary cation arises by subsequent rearrangement. The secondary cation is probably stabilized in the enzyme pocket by the proximity of an electron-rich aromatic ring.
Figure 27.15 MECHANISM
Mechanism of the conversion of 2,3-oxidosqualene to lanosterol. Four cationic cyclizations are followed by four rearrangements and a final loss of H+ from C9. The steroid numbering system is used for referring to specific positions in the intermediates (Section 27.6). Individual steps are explained in the text.
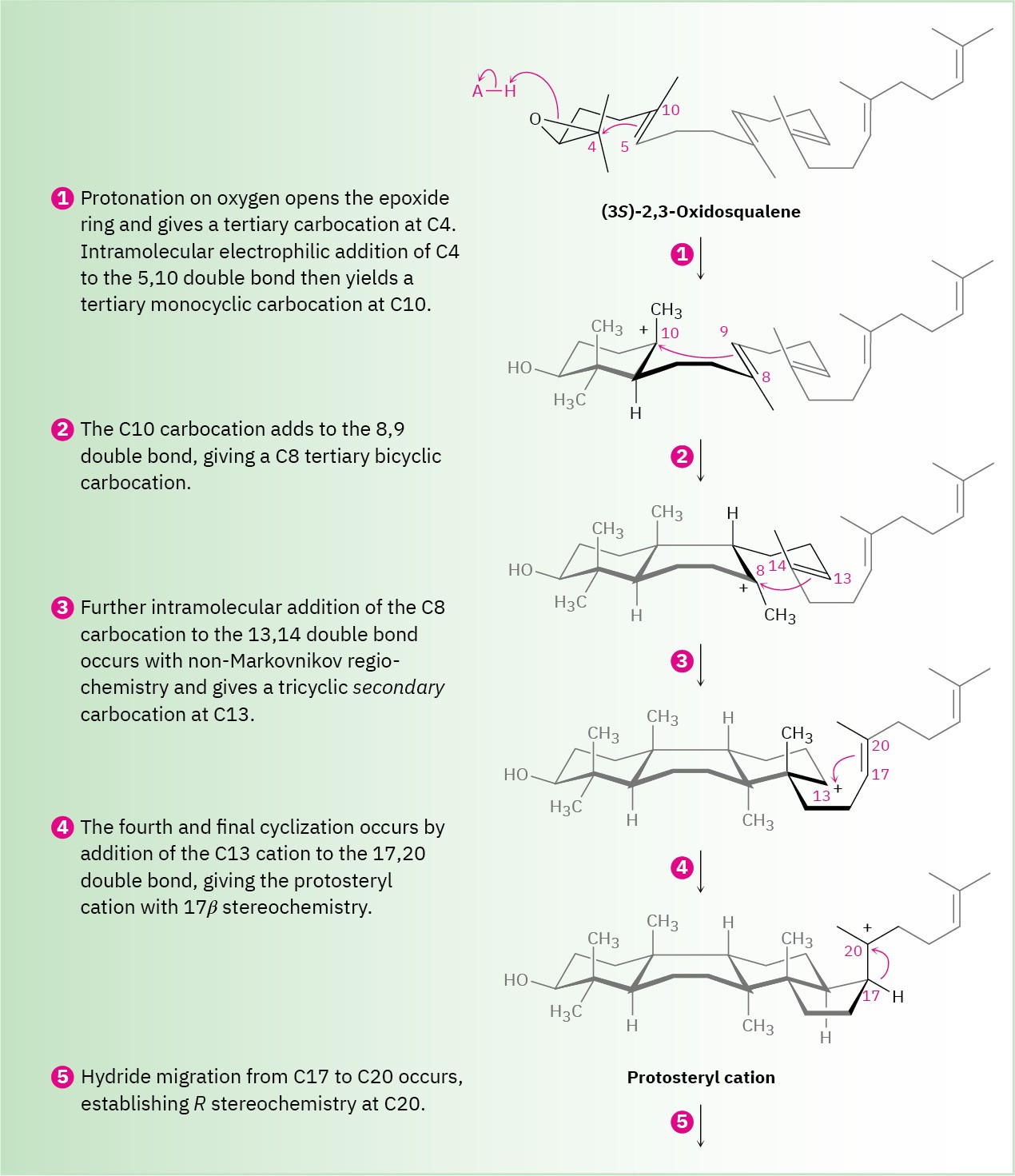
Figure 27.16
(Continued)
Step 4 of Figure 27.15: Final Cyclization
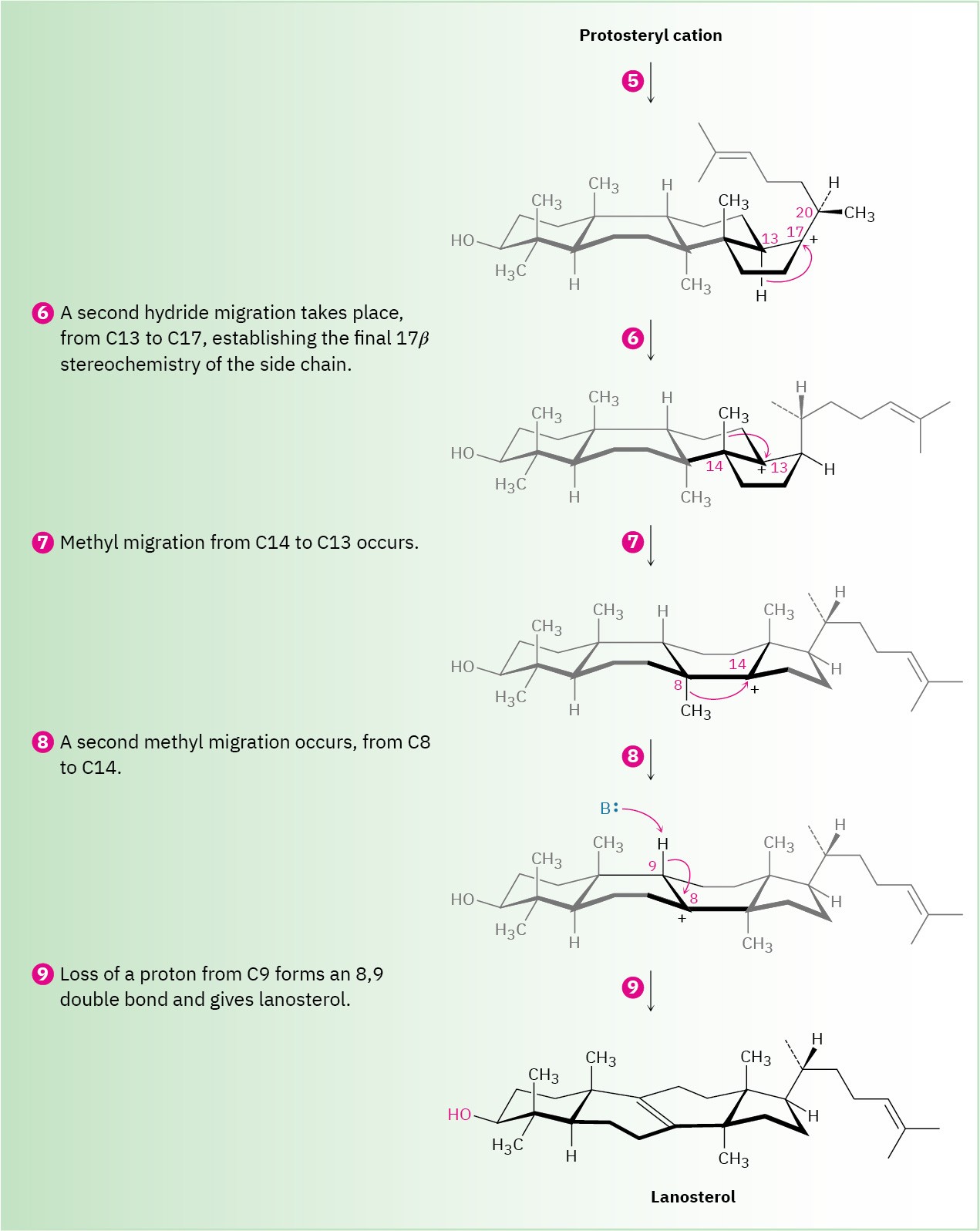
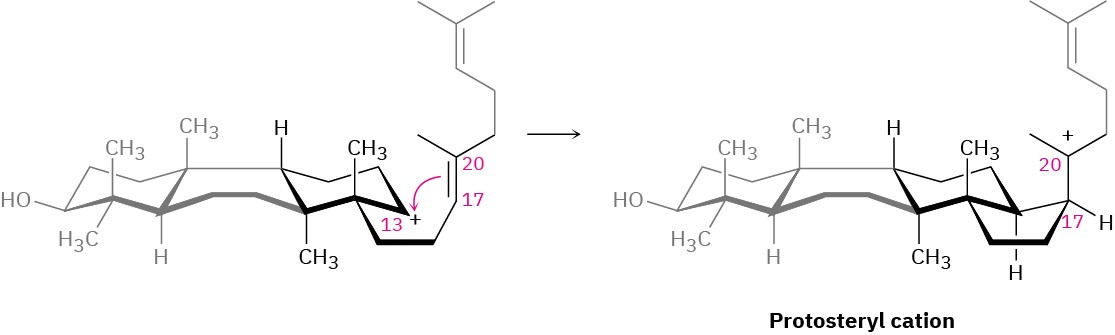
The fourth and last cyclization occurs in step 4 by addition of the cationic center at C13 to the 17,20 double bond, giving what is known as the protosteryl cation. The side-chain alkyl group at C17 has β (up) stereochemistry, although this stereochemistry is lost in step 5 and then reset in step 6.
Steps 5–9 of Figure 27.15: Carbocation Rearrangements
Once the tetracyclic carbon skeleton of lanosterol has been formed, a series of carbocation rearrangements occur (Section 7.11). The first rearrangement, hydride migration from C17 to C20, occurs in step 5 and results in establishment of R stereochemistry at C20 in the side chain. In step 6, a second hydride migration occurs from C13 to C17 on the α (bottom) face of the ring and reestablishes the 17β orientation of the side chain. Finally, two methyl migrations, the first from C14 to C13 on the top (β) face and the second from C8 to C14 on the bottom (α) face, place the positive charge at C8. A basic histidine residue in the enzyme then removes the neighboring β proton from C9 to give lanosterol.
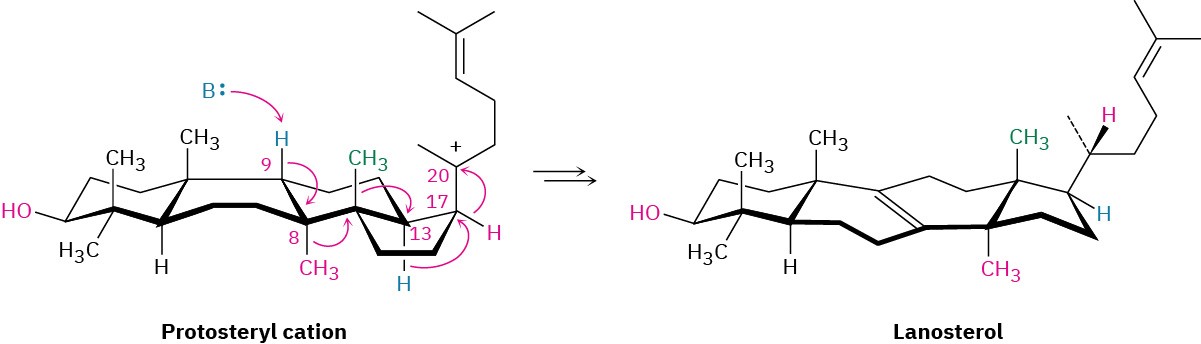
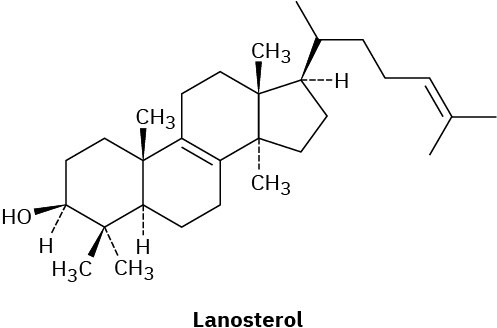
From lanosterol, the pathway for steroid biosynthesis continues on to yield cholesterol. Cholesterol then becomes a branch point, serving as the common precursor from which all other steroids are derived.
Problem 27-10
Compare the structures of lanosterol and cholesterol, and catalog the changes needed for the transformation shown.
Additional Problems 27 • Additional Problems 27 • Additional Problems Visualizing Chemistry Problem 27-11
The following model is that of cholic acid, a constituent of human bile. Locate the three hydroxyl groups, and identify each as axial or equatorial. Is cholic acid an A–B trans steroid or an A–B cis steroid?
Problem 27-12
Propose a biosynthetic pathway for the sesquiterpenoid helminthogermacrene from farnesyl diphosphate.
Problem 27-13
Identify the following fatty acid, and tell whether it is more likely to be found in peanut oil or in red meat:
Mechanism Problems
Problem 27-14
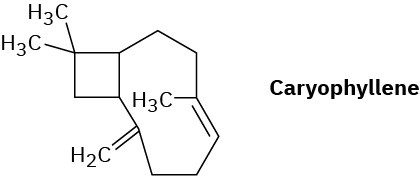
Propose a mechanistic pathway for the biosynthesis of caryophyllene, a substance found in clove oil.
Problem 27-15
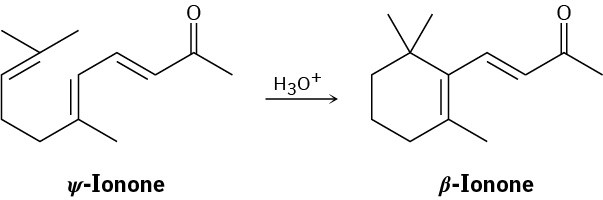
Suggest a mechanism by which ψ-ionone is transformed into β-ionone on treatment with acid.
Problem 27-16
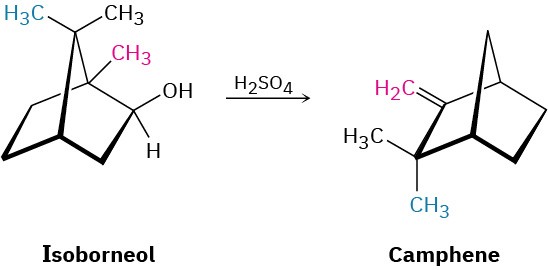
Isoborneol is converted into camphene on treatment with dilute sulfuric acid. Propose a mechanism for the reaction, which involves a carbocation rearrangement.
Fats, Oils, and Related Lipids
Problem 27-17
Fatty fish like salmon and albacore are rich in omega-3 fatty acids, which have a double bond three carbons in from the noncarboxyl end of the chain and have been shown to
lower blood cholesterol levels. Draw the structure of 5,8,11,14,17-eicosapentaenoic acid, a common example. (Eicosane = C20H42.)
Problem 27-18
Fats can be either optically active or optically inactive, depending on their structure. Draw the structure of an optically active fat that yields 2 equivalents of stearic acid and 1 equivalent of oleic acid on hydrolysis. Draw the structure of an optically inactive fat that yields the same products.
Problem 27-19
Spermaceti, a fragrant substance from sperm whales, was widely used in cosmetics until it was banned in 1976 to protect whales from extinction. Chemically, spermaceti is cetyl palmitate, the ester of cetyl alcohol (n-C16H33OH) with palmitic acid. Draw its structure.
Problem 27-20
Show the products you would expect to obtain from reaction of glyceryl trioleate with the following reagents:
(a)
Excess Br2 in CH2Cl2 (b)
H2/Pd (c)
NaOH/H2O
(d)
O3, then Zn/CH3CO2H (e)
LiAlH4, then H3O+ (f)
CH3MgBr, then H3O+ Problem 27-21
How would you convert oleic acid into the following substances? (a)
Methyl oleate (b)
Methyl stearate (c)
Nonanal (d)
Nonanedioic acid (e)
9-Octadecynoic acid (stearolic acid) (f)
2-Bromostearic acid (g)
18-Pentatriacontanone, CH3(CH2)16CO(CH2)16CH3 Problem 27-22
Plasmalogens are a group of lipids found in nerve and muscle cells. How do plasmalogens differ from fats?
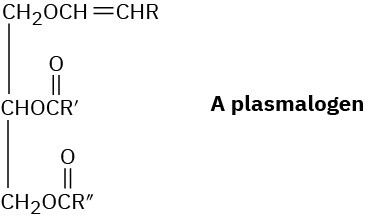
Problem 27-23
What products would you obtain from hydrolysis of a plasmalogen (Problem 27-22) with aqueous NaOH? With H3O+?
Problem 27-24
Cardiolipins are a group of lipids found in heart muscles. What products would be formed if all ester bonds, including phosphates, were saponified by treatment with aqueous NaOH?
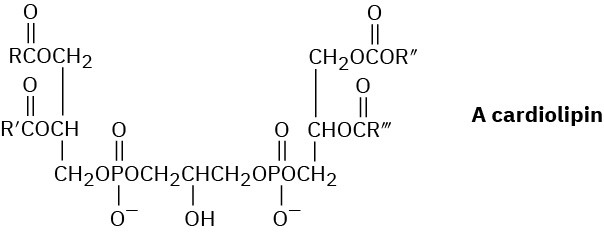
Problem 27-25
Stearolic acid, C18H32O2, yields stearic acid on catalytic hydrogenation and undergoes oxidative cleavage with ozone to yield nonanoic acid and nonanedioic acid. What is the structure of stearolic acid?
Problem 27-26
How would you synthesize stearolic acid (Problem 27-25) from 1-decyne and 1-chloro-7- iodoheptane?
Terpenoids and Steroids
Problem 27-27
Without proposing an entire biosynthetic pathway, draw the appropriate precursor, either geranyl diphosphate or farnesyl diphosphate, in a conformation that shows a likeness to each of the following terpenoids:
(a)
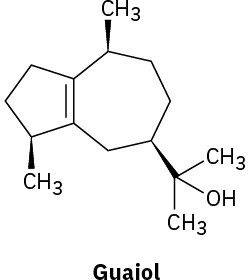
(b)
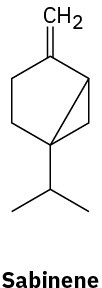
Problem 27-28
Indicate by asterisks the chirality centers present in each of the terpenoids shown in Problem 27-27. What is the maximum possible number of stereoisomers for each?
Problem 27-29
Assume that the three terpenoids in Problem 27-27 are derived biosynthetically from isopentenyl diphosphate and dimethylallyl diphosphate, each of which was isotopically labeled at the diphosphate-bearing carbon atom (C1). At what positions would the terpenoids be isotopically labeled?
Problem 27-30
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material for the biosynthesis of mevalonate, as shown in Figure 27.8. At what positions in mevalonate would the isotopic label appear?
Problem 27-31
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material and that the mevalonate pathway is followed. Identify the positions in α-cadinol where the label would appear.
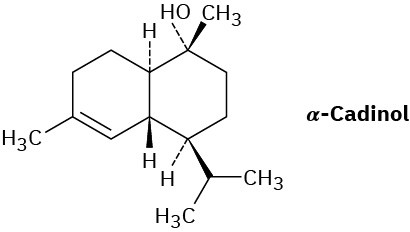
Problem 27-32
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material and that the mevalonate pathway is followed. Identify the positions in squalene where the label would appear.
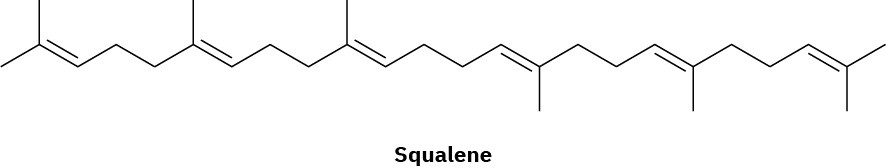
Problem 27-33
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material and that the mevalonate pathway is followed. Identify the positions in lanosterol where the label would appear.
General Problems
Problem 27-34
Flexibilene, a compound isolated from marine coral, is the first known terpenoid to contain a 15-membered ring. What is the structure of the acyclic biosynthetic precursor of flexibilene? Show the mechanistic pathway for the biosynthesis.
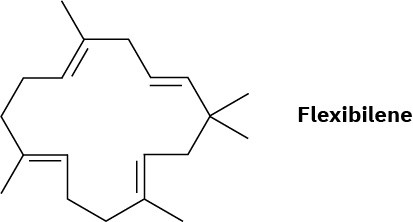
Problem 27-35
Draw the most stable chair conformation of dihydrocarvone.
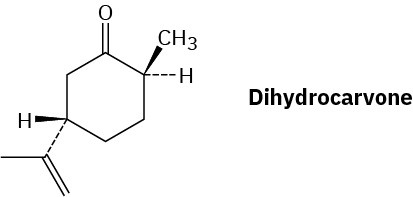
Problem 27-36
Draw the most stable chair conformation of menthol, and label each substituent as axial or equatorial.
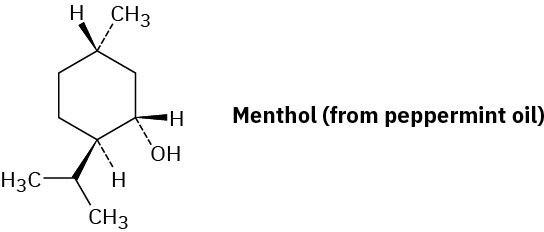
Problem 27-37
As a general rule, equatorial alcohols are esterified more readily than axial alcohols. What product would you expect to obtain from reaction of the following two compounds with 1 equivalent of acetic anhydride?
(a)
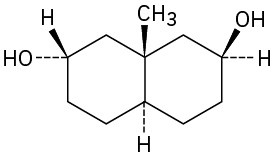
(b)
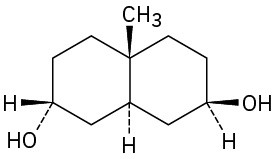
Problem 27-38
Propose a mechanistic pathway for the biosynthesis of isoborneol. A carbocation rearrangement is needed at one point in the scheme.
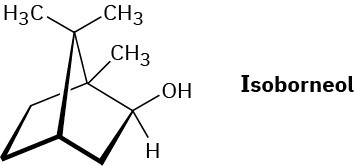
Problem 27-39
Digitoxigenin is a heart stimulant obtained from the purple foxglove Digitalis purpurea and used in the treatment of heart disease. Draw the three-dimensional conformation of digitoxigenin, and identify the two –OH groups as axial or equatorial.
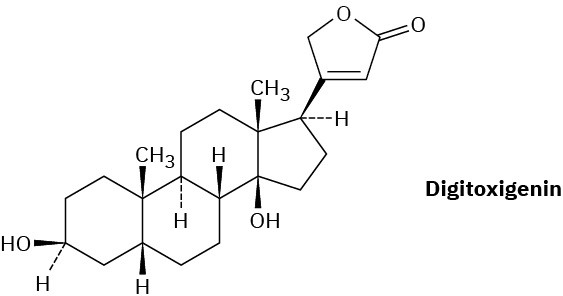
Problem 27-40
What product would you obtain by reduction of digitoxigenin (Problem 27-39) with LiAlH4? By oxidation with the Dess–Martin periodinane?
Problem 27-41
Vaccenic acid, C18H34O2, is a rare fatty acid that gives heptanal and 11-oxoundecanoic acid [OHC(CH2)9CO2H] on ozonolysis followed by zinc treatment. When allowed to react with CH2I2/Zn(Cu), vaccenic acid is converted into lactobacillic acid. What are the structures of vaccenic and lactobacillic acids?
Problem 27-42
Eleostearic acid, C18H30O2, is a rare fatty acid found in the tung oil used for finishing furniture. On ozonolysis followed by treatment with zinc, eleostearic acid furnishes one part pentanal, two parts glyoxal (OHC–CHO), and one part 9-oxononanoic acid [OHC(CH2)7CO2H]. What is the structure of eleostearic acid?
Problem 27-43
Diterpenoids are derived biosynthetically from geranylgeranyl diphosphate (GGPP), which is itself biosynthesized by reaction of farnesyl diphosphate with isopentenyl diphosphate. Show the structure of GGPP, and propose a mechanism for its biosynthesis from FPP and IPP.
Problem 27-44
Diethylstilbestrol (DES) has estrogenic activity even though it is structurally unrelated to steroids. Once used as an additive in animal feed, DES has been implicated as a causative agent in several types of cancer. Show how DES can be drawn so that it is sterically similar to estradiol.
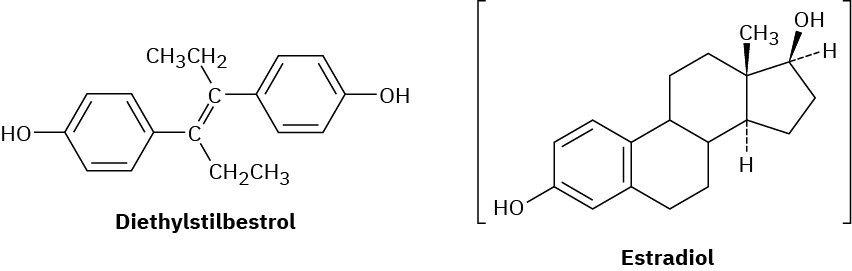
Problem 27-45
Cembrene, C20H32, a diterpenoid hydrocarbon isolated from pine resin, has a UV absorption at 245 nm, but dihydrocembrene (C20H34), the product of hydrogenation with 1 equivalent of H2, has no UV absorption. On exhaustive hydrogenation, 4 equivalents of H2 react, and octahydrocembrene, C20H40, is produced. On ozonolysis of cembrene, followed by treatment of the ozonide with zinc, four carbonyl-containing products are obtained:
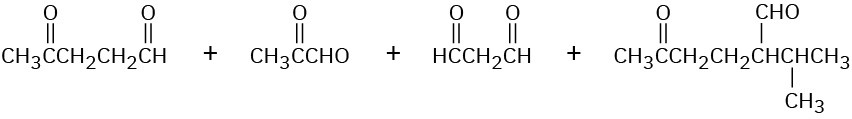
Propose a structure for cembrene that is consistent with its formation from geranylgeranyl diphosphate.
Problem 27-46
α-Fenchone is a pleasant-smelling terpenoid isolated from oil of lavender. Propose a pathway for the formation of α-fenchone from geranyl diphosphate. A carbocation rearrangement is required.
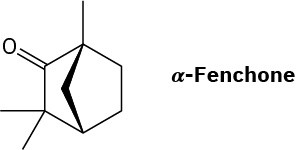
Problem 27-47
Fatty acids are synthesized by a multistep route that starts with acetate. The first step is a reaction between protein-bound acetyl and malonyl units to give a protein-bound 3- ketobutyryl unit. Show the mechanism, and tell what kind of reaction is occurring.
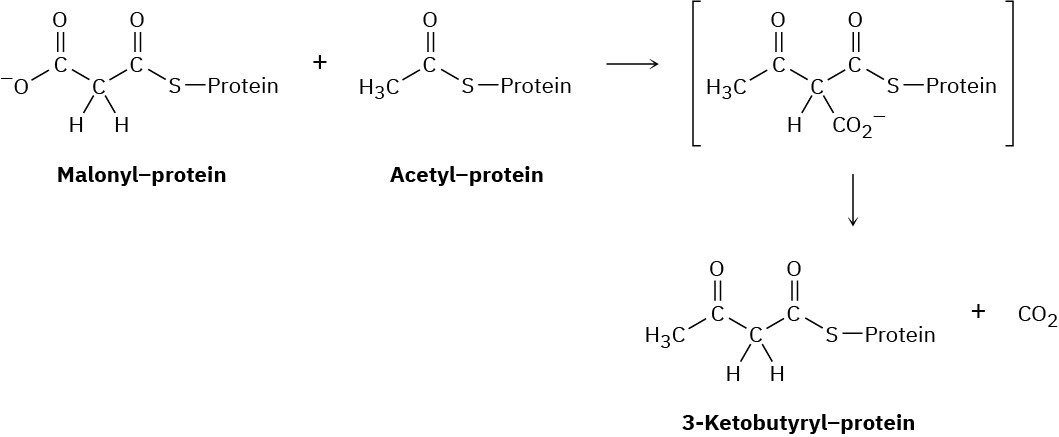
Problem 27-48
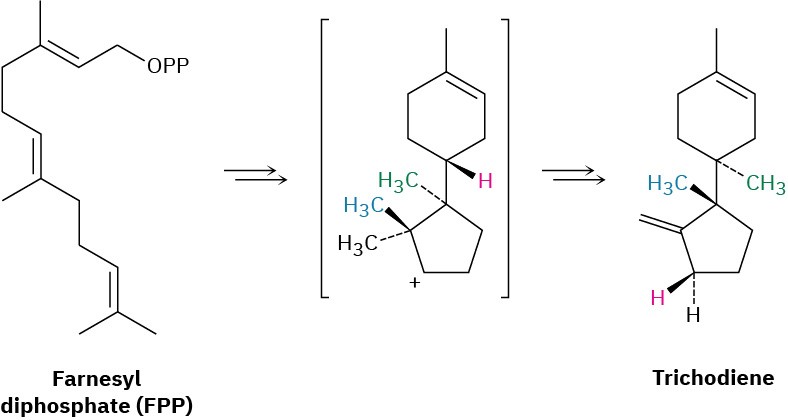
Propose a mechanism for the biosynthesis of the sesquiterpenoid trichodiene from farnesyl diphosphate. The process involves cyclization to give an intermediate secondary carbocation, followed by several carbocation rearrangements.