Summary
We’ve continued the coverage of aromatic molecules in this chapter, shifting focus to concentrate on reactions. In particular, we’ve looked at the relationship between aromatic structure and reactivity, a relationship critical to understanding how numerous biological molecules and pharmaceutical agents are synthesized and why they behave as they do.
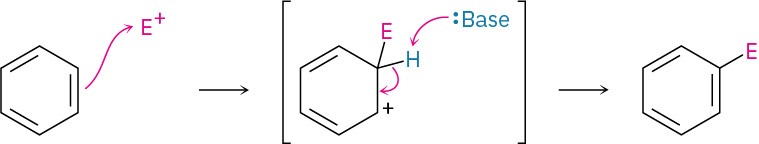
An electrophilic aromatic substitution reaction takes place in two steps—initial reaction of an electrophile, E+, with the aromatic ring, followed by loss of H+ from the resonance-stabilized carbocation intermediate to regenerate the aromatic ring.
Many variations of the reaction can be carried out, including halogenation, nitration, and sulfonation. Friedel–Crafts alkylation and acylation reactions, which involve reaction of an aromatic ring with carbocation electrophiles, are particularly useful. They are limited, however, by the fact that the aromatic ring must be at least as reactive as a halobenzene. In addition, polyalkylation and carbocation rearrangements often occur in Friedel–Crafts alkylation.
Substituents on the benzene ring affect both the reactivity of the ring toward further substitution and the orientation of that substitution. Groups can be classified as ortho- and para-directing activators, ortho- and para-directing deactivators, or meta-directing deactivators. Substituents influence aromatic rings by a combination of resonance and inductive effects. Resonance effects are transmitted through π bonds; inductive effects are transmitted through σ bonds.
Halobenzenes undergo nucleophilic aromatic substitution through either of two mechanisms. If the halobenzene has a strongly electron-withdrawing substituent in the ortho or para position, substitution occurs by addition of a nucleophile to the ring, followed by elimination of halide from the intermediate anion. If the halobenzene is not activated by an electron-withdrawing substituent, substitution can occur by elimination of HX to give a benzyne, followed by addition of a nucleophile.
The benzylic position of an alkylbenzene can be brominated by reaction with N– bromosuccinimide, and the entire side chain can be degraded to a carboxyl group by oxidation with aqueous KMnO4. Aromatic rings can also be reduced to cyclohexanes by hydrogenation over a platinum or rhodium catalyst, and aryl alkyl ketones are reduced to alkylbenzenes by hydrogenation over a platinum catalyst.

