7.7 Electrophilic Addition Reactions of Alkenes
Before beginning a detailed discussion of alkene reactions, let’s review briefly some conclusions from the previous chapter. We said in Section 6.5 that alkenes behave as nucleophiles (Lewis bases) in polar reactions, donating a pair of electrons from their electron-rich C═C bond to an electrophile (Lewis acid). For example, reaction of 2- methylpropene with HBr yields 2-bromo-2-methylpropane. A careful study of this and similar reactions by the British chemist Christopher Ingold and others in the 1930s led to the generally accepted mechanism shown in Figure 7.8 for an electrophilic addition reaction.
Figure 7.8 MECHANISM
Mechanism of the electrophilic addition of HBr to 2-methylpropene. The reaction occurs in two steps, protonation and bromide addition, and involves a carbocation intermediate.
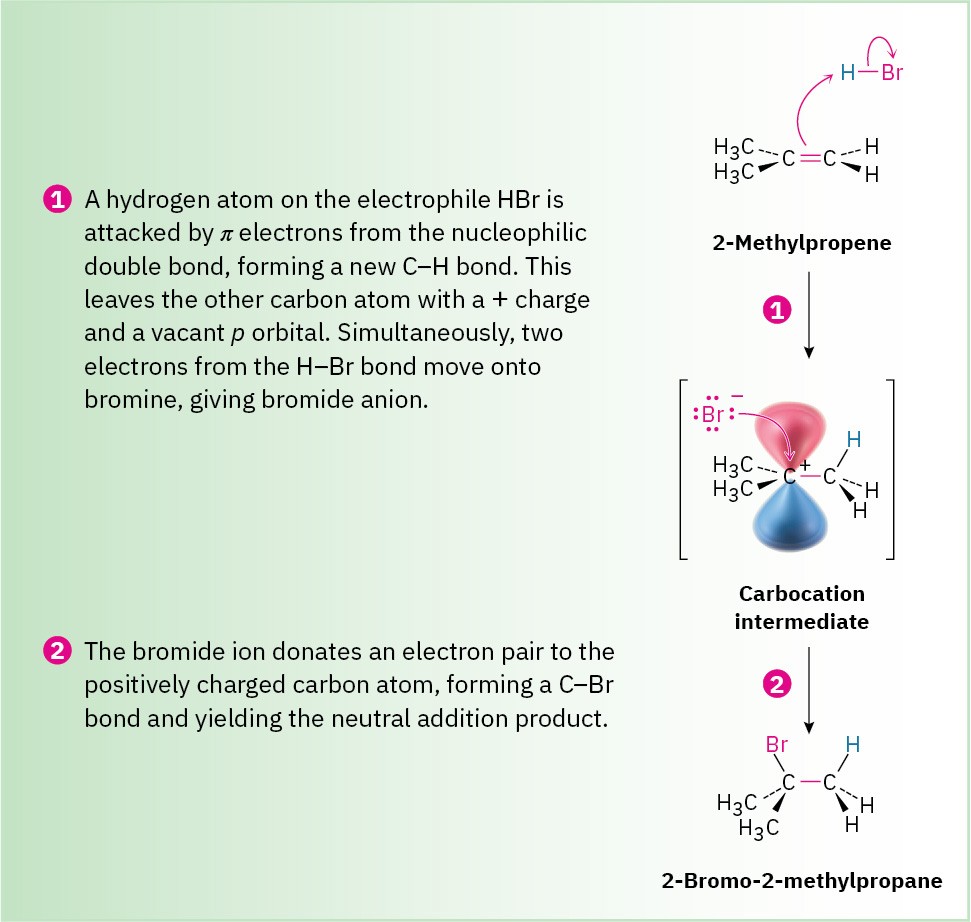
The reaction begins with an attack on the hydrogen of the electrophile HBr by the electrons of the nucleophilic π bond. Two electrons from the π bond form a new σ bond between the entering hydrogen and an alkene carbon, as shown by the curved arrow at the top of Figure
7.8. The resulting carbocation intermediate is itself an electrophile, which can accept an electron pair from nucleophilic Br− ion to form a C−Br bond and yield a neutral addition product.
An energy diagram for the overall electrophilic addition reaction (Figure 7.9) has two peaks (transition states) separated by a valley (carbocation intermediate). The energy level of the intermediate is higher than that of the starting alkene, but the reaction as a whole is exergonic (negative ΔG°). The first step, protonation of the alkene to yield the intermediate cation, is relatively slow. But once the cation intermediate is formed, it rapidly reacts to yield the final alkyl bromide product. The relative rates of the two steps are indicated in Figure 7.9 by the fact that ΔG1‡ is larger than ΔG2‡.
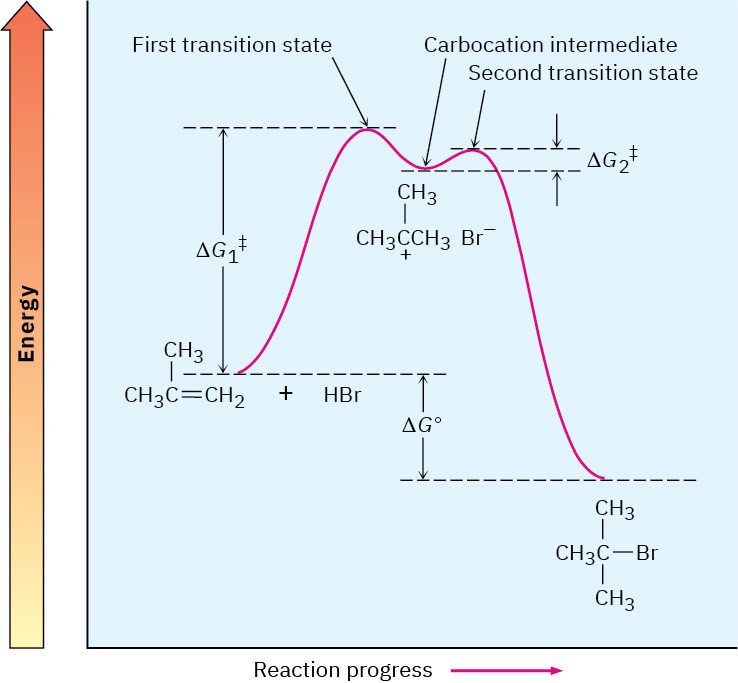
Figure 7.9 Energy diagram for the two-step electrophilic addition of HBr to 2- methylpropene. The first step is slower than the second step.
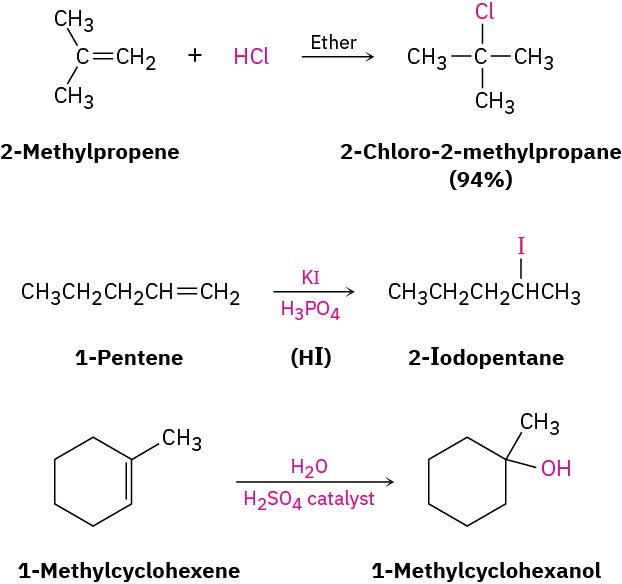
Electrophilic addition to alkenes is successful not only with HBr but with HCl, HI, and H2O as well. Note that HI is usually generated in the reaction mixture by treating potassium iodide with phosphoric acid and that a strong acid catalyst is needed for the addition of water.
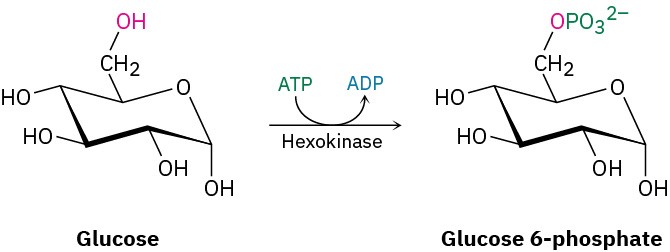
Writing Organic ReactionsThis is a good place to mention that the equations for organic reactions are sometimes written in different ways to emphasize different points. In describing a laboratory process, for instance, the reaction of 2-methylpropene with HCl might be written in the format A + B⟶ C to emphasize that both reactants are equally important for the purposes of the discussion. The solvent and notes about other reaction conditions such as temperature are written either above or below the reaction arrow.
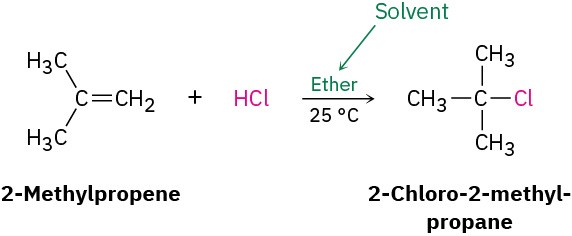
Alternatively, we might write the same reaction in a format to emphasize that 2- methylpropene is the reactant whose chemistry is of greater interest. The second reactant, HCl, is placed above the reaction arrow together with notes about solvent and reaction conditions.
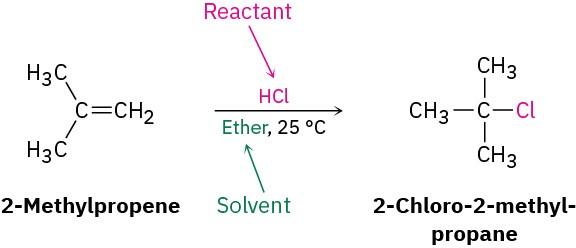
In describing a biological process, the reaction is almost always written to show only the structures of the primary reactant and product, while abbreviating the structures of various biological “reagents” and by-products with a curved arrow that intersects the
straight reaction arrow. As discussed in Section 6.11, the reaction of glucose with ATP to give glucose 6-phosphate plus ADP would then be written as