11.1 The Discovery of Nucleophilic Substitution Reactions
Discovery of the nucleophilic substitution reaction of alkyl halides dates back to work carried out by the German chemist Paul Walden in 1896. Walden found that the pure enantiomeric (+)- and (–)-malic acids could be interconverted through a series of simple substitution reactions. When Walden treated (–)-malic acid with PCl5, he isolated (+)- chlorosuccinic acid. This, on treatment with wet Ag2O, gave (+)-malic acid. Similarly, reaction of (+)-malic acid with PCl5 gave (–)-chlorosuccinic acid, which was converted into (–)-malic acid when treated with wet Ag2O. The full cycle of reactions is shown in Figure 11.2.
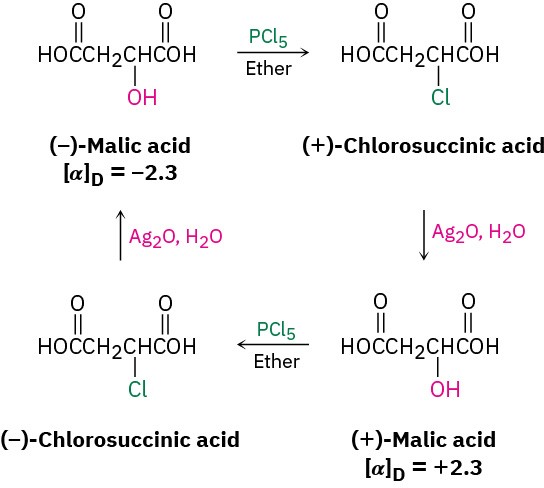
Figure 11.2Walden’s cycle of reactions interconverting (+)- and (–)-malic acids.
At the time, the results were astonishing. The eminent chemist Emil Fischer called Walden’s discovery “the most remarkable observation made in the field of optical activity since the fundamental observations of Pasteur.” Because (–)-malic acid was converted into (+)-malic acid, some reactions in the cycle must have occurred with a change, or inversion, of configuration at the chirality center. But which ones, and how? (Remember from Section
5.5 that the direction of light rotation and the configuration of a chirality center aren’t directly related. You can’t tell by looking at the sign of rotation whether a change in configuration has occurred during a reaction.)
Today, we refer to the transformations taking place in Walden’s cycle as nucleophilic substitution reactions because each step involves the substitution of one nucleophile (chloride ion, Cl–, or hydroxide ion, HO–) by another. Nucleophilic substitution reactions are one of the most common and versatile reaction types in organic chemistry.

Following the work of Walden, further investigations were undertaken during the 1920s and 1930s to clarify the mechanism of nucleophilic substitution reactions and to find out
how inversions of configuration occur. Among the first series studied was one that interconverted the two enantiomers of 1-phenyl-2-propanol (Figure 11.3). Although this particular series of reactions involves nucleophilic substitution of an alkyl para– toluenesulfonate (called a tosylate) rather than an alkyl halide, exactly the same type of reaction is involved as that studied by Walden. For all practical purposes, the entire tosylate group acts as if it were simply a halogen substituent. (In fact, when you see a tosylate substituent in a molecule, do a mental substitution and tell yourself that you’re dealing with an alkyl halide.)
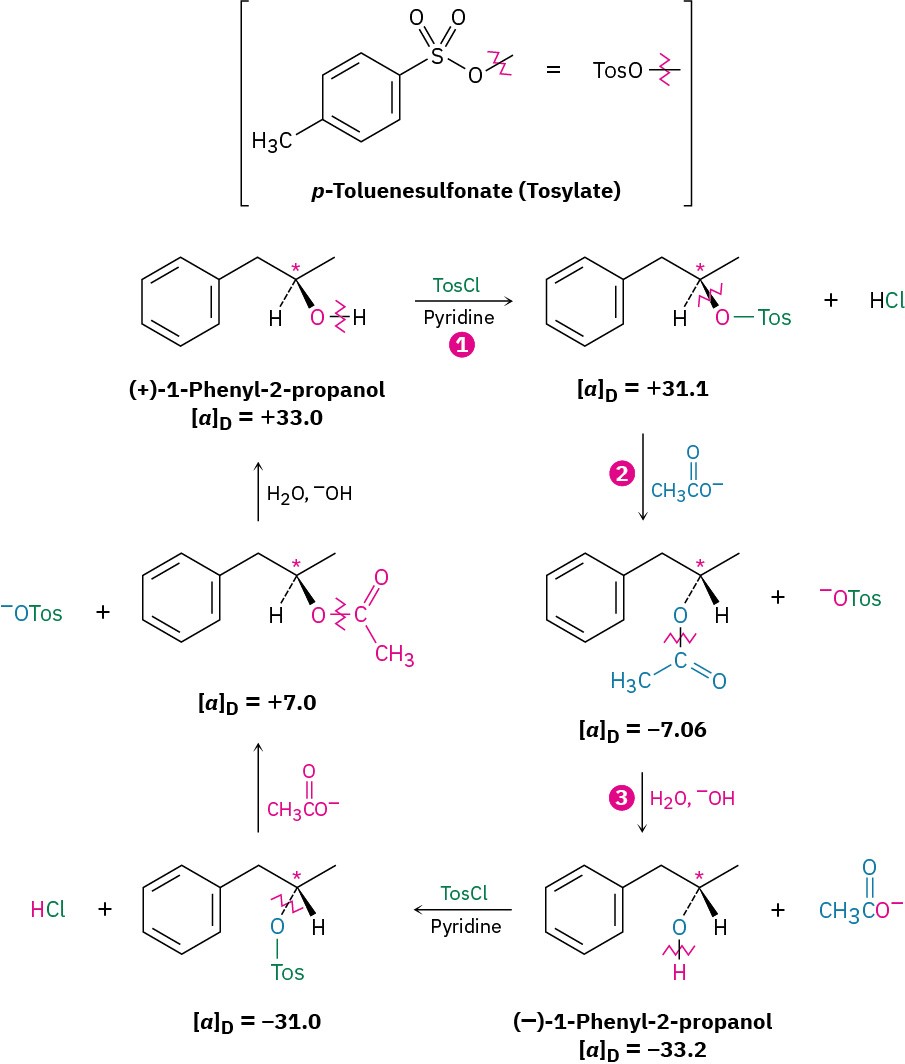
Figure 11.3 A Walden cycle interconverting (+) and (–) enantiomers of 1-phenyl-2- propanol. Chirality centers are marked by asterisks, and the bonds broken in each reaction are indicated by red wavy lines. The inversion of chirality occurs in step 2, where acetate ion substitutes for tosylate ion.
In the three-step reaction sequence shown in Figure 11.3, (+)-1-phenyl-2-propanol is interconverted with its (–) enantiomer, so at least one of the three steps must involve an inversion of configuration at the chirality center. Step 1, formation of a tosylate, occurs by breaking the O–H bond of the alcohol rather than the C–O bond to the chiral carbon, so the
configuration around the carbon is unchanged. Similarly, step 3, hydroxide-ion cleavage of the acetate, takes place without breaking the C–O bond at the chirality center. Thus, the inversion of stereochemical configuration must take place in step 2, the nucleophilic substitution of tosylate ion by acetate ion.
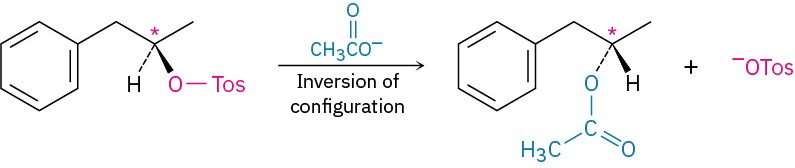
From this and nearly a dozen other series of similar reactions, researchers concluded that the nucleophilic substitution reaction of a primary or secondary alkyl halide or tosylate always proceeds with inversion of configuration. (Tertiary alkyl halides and tosylates, as we’ll see shortly, give different stereochemical results and react by a different mechanism than the primary and secondary ones.)
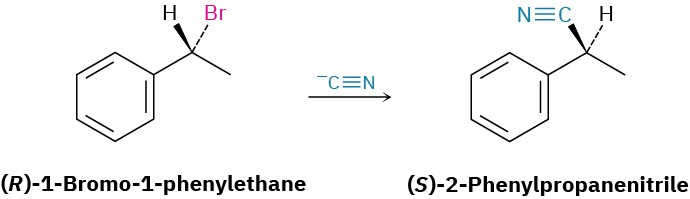
Worked Example 11.1Predicting the Stereochemistry of a Nucleophilic Substitution ReactionWhat product would you expect from a nucleophilic substitution reaction of (R)-1-bromo- 1-phenylethane with cyanide ion, !C≡N, as nucleophile? Show the stereochemistry of both reactant and product, assuming that inversion of configuration occurs.
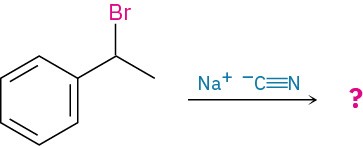 StrategyDraw the R enantiomer of the reactant, and then change the configuration of the chirality center while replacing the –Br with a –CN.Solution
StrategyDraw the R enantiomer of the reactant, and then change the configuration of the chirality center while replacing the –Br with a –CN.Solution
Problem 11-1
What product would you expect from a nucleophilic substitution reaction of (S)-2- bromohexane with acetate ion, CH3CO2–? Assume that inversion of configuration occurs, and show the stereochemistry of both the reactant and product.

