13.7 1H NMR Spectroscopy and Proton Equivalence
Because each electronically distinct hydrogen in a molecule has its own unique absorption, one use of 1H NMR is to find out how many kinds of electronically nonequivalent hydrogens are present. In the 1H NMR spectrum of methyl acetate shown previously in Figure 13.4a, for instance, there are two signals, corresponding to the two kinds of nonequivalent protons present, CH3C═O protons and –OCH3 protons.
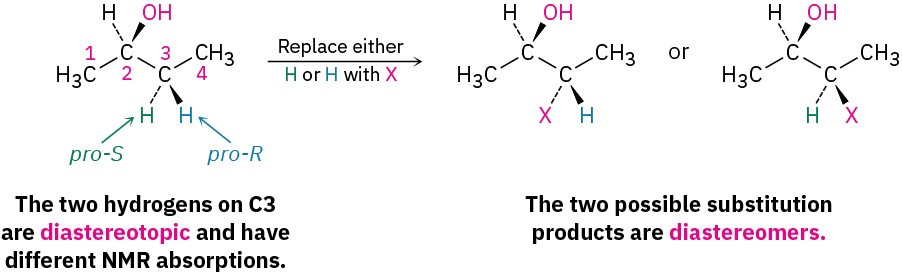
For relatively small molecules, a quick look at the structure is often enough to decide how many kinds of protons are present and thus how many NMR absorptions might appear. If in doubt, though, the equivalence or nonequivalence of two protons can be determined by comparing the structures that would be formed if each hydrogen were replaced by an X group. There are four possibilities.
- One possibility is that the protons are chemically unrelated and thus nonequivalent. If so, the products formed on substitution of H by X would be different constitutional isomers. In butane, for instance, the –CH3 protons are different from the –CH2– protons. They therefore give different products on substitution by X than the –CH2 protons and would likely show different NMR absorptions.
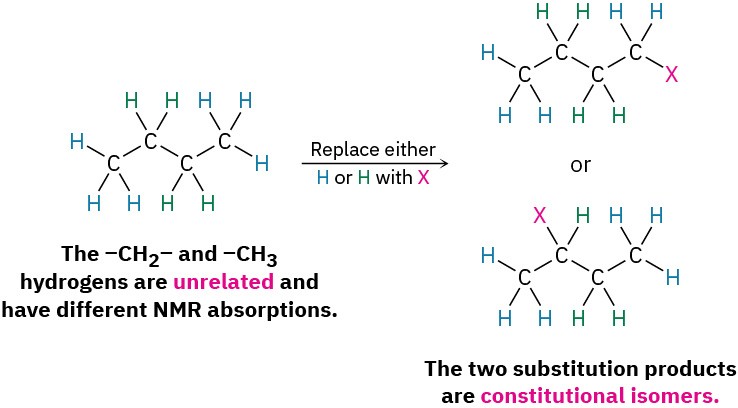
- A second possibility is that the protons are chemically identical and thus electronically equivalent. If so, the same product would be formed regardless of which H is substituted by X. In butane, for instance, the six –CH3 hydrogens on C1 and C4 are identical, would give the identical structure on substitution by X, and would show an identical NMR absorption. Such protons are said to be homotopic.
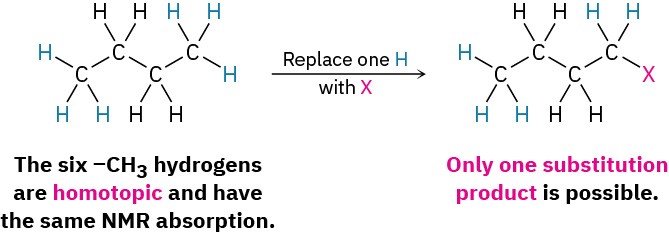
- The third possibility is a bit more subtle. Although they might at first seem homotopic, the two –CH2– hydrogens on C2 in butane (and the two –CH2– hydrogens on C3) are in fact not identical. Substitution by X of a hydrogen at C2 (or C3) would
form a new chirality center, so different enantiomers (Section 5.1) would result, depending on whether the pro-R or pro-S hydrogen had been substituted (Section 5.11). Such hydrogens, whose substitution by X would lead to different enantiomers, are said to be enantiotopic. Enantiotopic hydrogens, even though not identical, are nevertheless electronically equivalent and thus have the same NMR absorption.
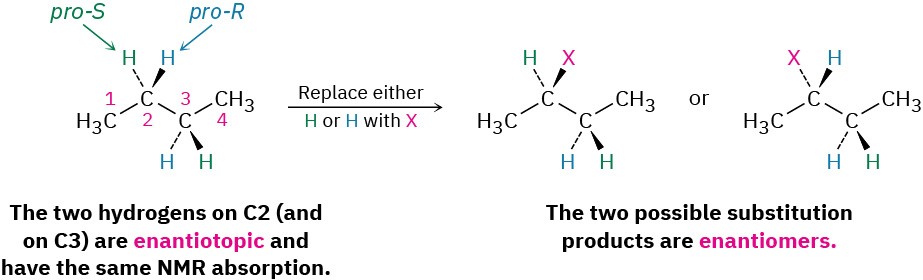
- The fourth possibility arises in chiral molecules, such as (R)-2-butanol. The two – CH2– hydrogens at C3 are neither homotopic nor enantiotopic. Because substitution of a hydrogen at C3 would form a second chirality center, different diastereomers (Section 5.6) would result, depending on whether the pro-R or pro-S hydrogen had been substituted. Such hydrogens, whose substitution by X leads to different diastereomers, are said to be diastereotopic. Diastereotopic hydrogens are neither chemically nor electronically equivalent. They are completely different and would likely show different NMR absorptions.
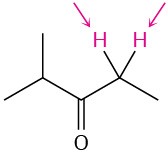
Problem 13-12
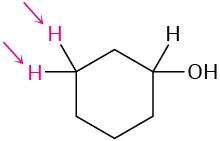
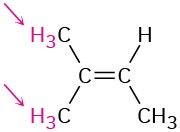
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, or diastereotopic:
(a)
(b)
(c)
(d)
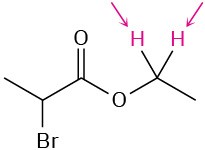
(e)
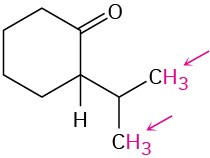
(f)
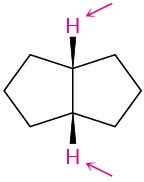
Problem 13-13
How many kinds of electronically nonequivalent protons are present in each of the following compounds, and thus how many NMR absorptions might you expect in each?
(a) CH3CH2Br
(b) CH3OCH2CH(CH3)2
(c) CH3CH2CH2NO2
(d) Methylbenzene (e)
- Methyl-1-butene
(f)
cis-3-Hexene Problem 13-14
How many absorptions would you expect (S)-malate, an intermediate in carbohydrate metabolism, to have in its 1H NMR spectrum? Explain.