18.6 Crown Ethers
Crown ethers, discovered in the early 1960s by Charles Pedersen at the DuPont Company, are a relatively recent addition to the ether family. They are named according to the general format x-crown-y, where x is the total number of atoms in the ring and y is the number of oxygen atoms. Thus, 18-crown-6 ether is an 18-membered ring containing 6 ether oxygen atoms. Note the size and negative (red) character of the crown ether cavity in the following electrostatic potential map.
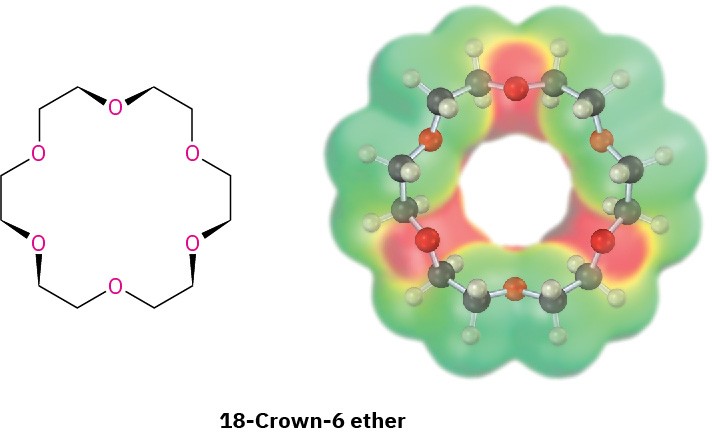
The importance of crown ethers stems from their ability to sequester specific metal cations in the center of the polyether cavity. 18-Crown-6, for example, binds strongly with potassium ion. As a result, a solution of 18-crown-6 in a nonpolar organic solvent will dissolve many potassium salts. Potassium permanganate, KMnO4, dissolves in toluene in the presence of 18-crown-6, for instance, and the resulting solution is a valuable reagent for oxidizing alkenes.
The effect of using a crown ether to dissolve an inorganic salt in a hydrocarbon or ether solvent is similar to the effect of dissolving the salt in a polar aprotic solvent such as DMSO, DMF, or HMPA (Section 11.3). In both cases, the metal cation is strongly solvated, leaving the anion bare. Thus, the SN2 reactivity of an anion is tremendously enhanced in the presence of a crown ether.
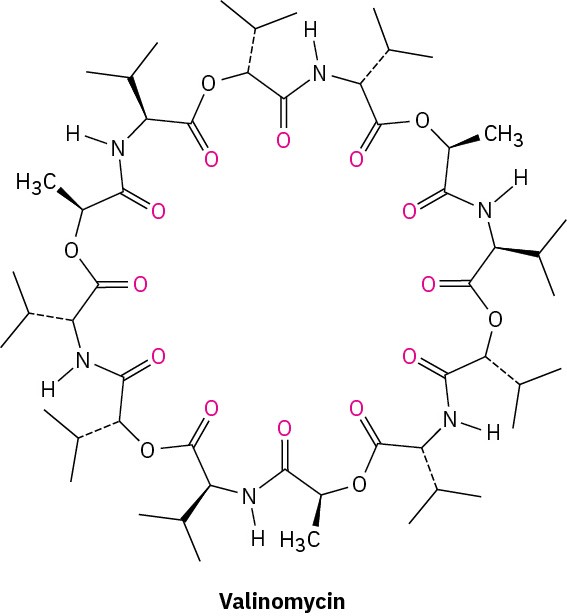
Although crown ethers do not occur naturally, a group of compounds called ionophores have similar ion-binding properties. Produced by various microorganisms, ionophores are fat-soluble molecules that bind to specific ions and facilitate transport of the ions through biological membranes. The antibiotic valinomycin, for instance, binds specifically to K+ ions with a ten-thousandfold selectivity over Na+.
Problem 18-15
15-Crown-5 and 12-crown-4 ethers complex Na+ and Li+, respectively. Make models of these crown ethers, and compare the sizes of the cavities.

