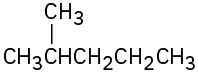19.9 Nucleophilic Addition of Hydrazine: The Wolff–Kishner Reaction
A useful variant of the imine-forming reaction just discussed involves the treatment of an aldehyde or ketone with hydrazine, H2N–NH2, in the presence of KOH. Called the Wolff– Kishner reaction, the process is a useful and general method for converting an aldehyde or ketone into an alkane, R!C=O → R!CH!.
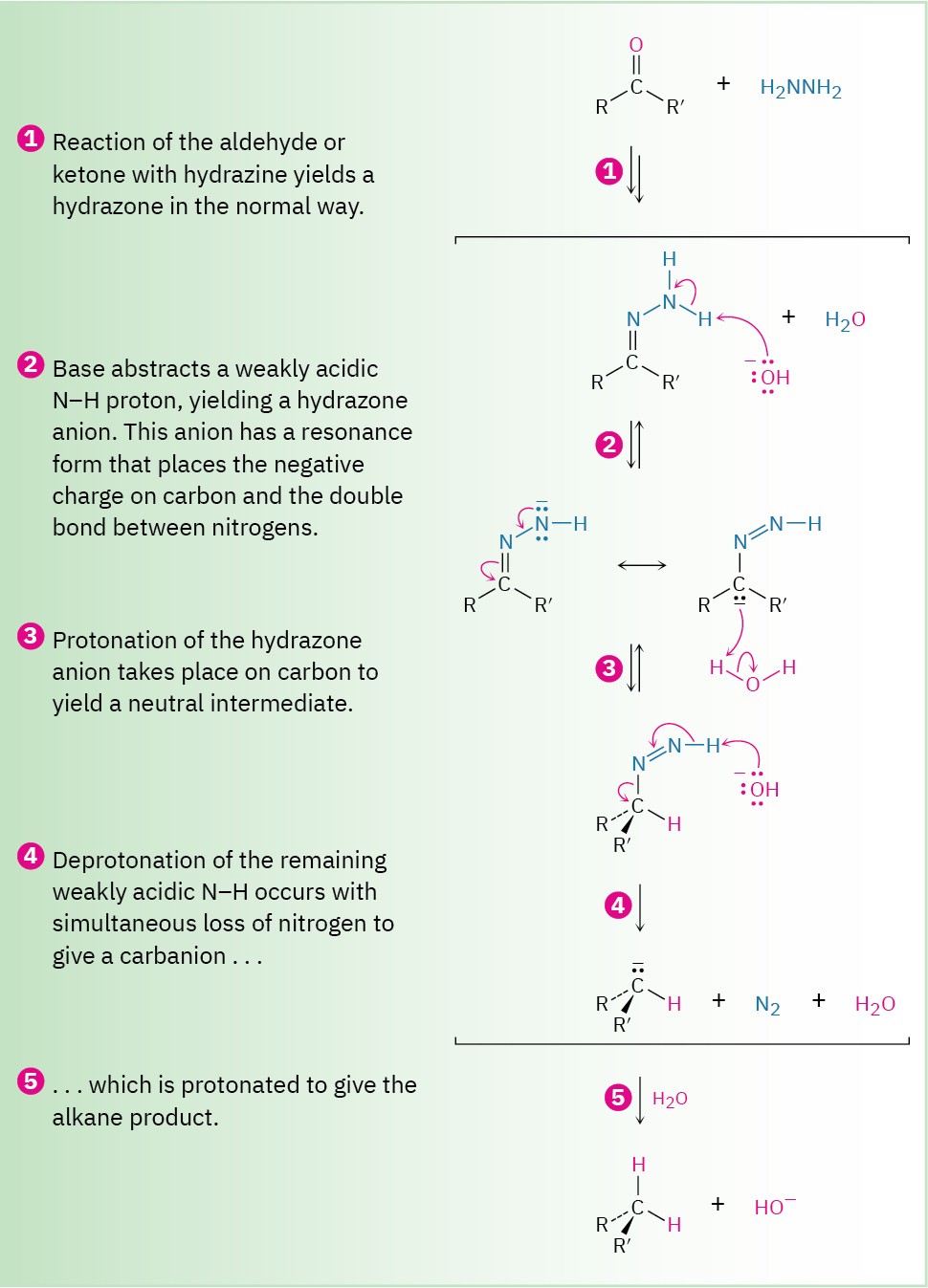
As shown in Figure 19.10, the Wolff–Kishner reaction involves formation of a hydrazone intermediate, R!C═N–NH!, followed by base-catalyzed double-bond migration, loss of N2 gas to give a carbanion, and protonation to give the alkane product. The double-bond migration takes place when a base removes one of the weakly acidic NH protons in step 2 to generate a hydrazone anion, which has an allylic resonance structure that places the double bond between nitrogens and the negative charge on carbon. Reprotonation then occurs on carbon to generate the double-bond rearrangement product. The next step—loss of nitrogen and formation of an alkyl anion—is driven by the large thermodynamic stability of the N2 molecule.
Figure 19.10 MECHANISM
Mechanism for the Wolff–Kishner reduction of an aldehyde or ketone to yield an alkane.
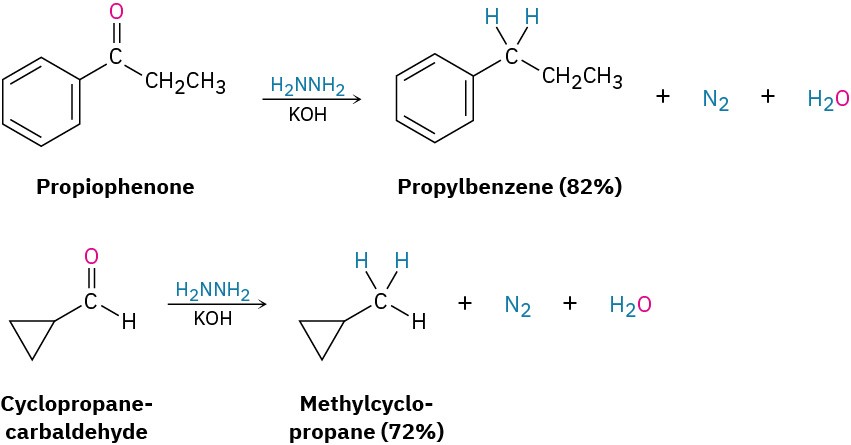
Note that the Wolff–Kishner reduction accomplishes the same overall transformation as the catalytic hydrogenation of an acylbenzene to yield an alkylbenzene (Section 16.10). The Wolff–Kishner reduction is more general and more useful than catalytic hydrogenation, however, because it works well with both alkyl and aryl ketones.
Problem 19-13
Show how you could prepare the following compounds from 4-methyl-3-penten-2-one, (CH3)2C=CHCOCH3.
(a)
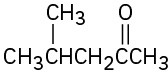
(b)
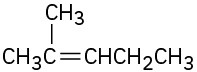
(c)