27.4 Prostaglandins and Other Eicosanoids
Prostaglandins are a group of C20 lipids that contain a five-membered ring with two long side chains. First isolated in the 1930s by Ulf von Euler at the Karolinska Institute in Sweden, much of the structural and chemical work on prostaglandins was carried out by Sune Bergström and Bengt Samuelsson. All three shared Nobel Prizes for their work. The name prostaglandin derives from the fact that the compounds were first isolated from sheep prostate glands, but they have subsequently been found to be present in small amounts in all body tissues and fluids.
The several dozen known prostaglandins have an extraordinarily wide range of biological effects. Among their many properties, they can lower blood pressure, affect blood platelet aggregation during clotting, lower gastric secretions, control inflammation, affect kidney function, affect reproductive systems, and stimulate uterine contractions during childbirth.
Prostaglandins, together with related compounds called thromboxanes and leukotrienes, make up a class of compounds called eicosanoids because they are derived biologically from 5,8,11,14-eicosatetraenoic acid, or arachidonic acid (Figure 27.4). Prostaglandins (PG) have a cyclopentane ring with two long side chains; thromboxanes (TX) have a six- membered, oxygen-containing ring; and leukotrienes (LT) are acyclic.
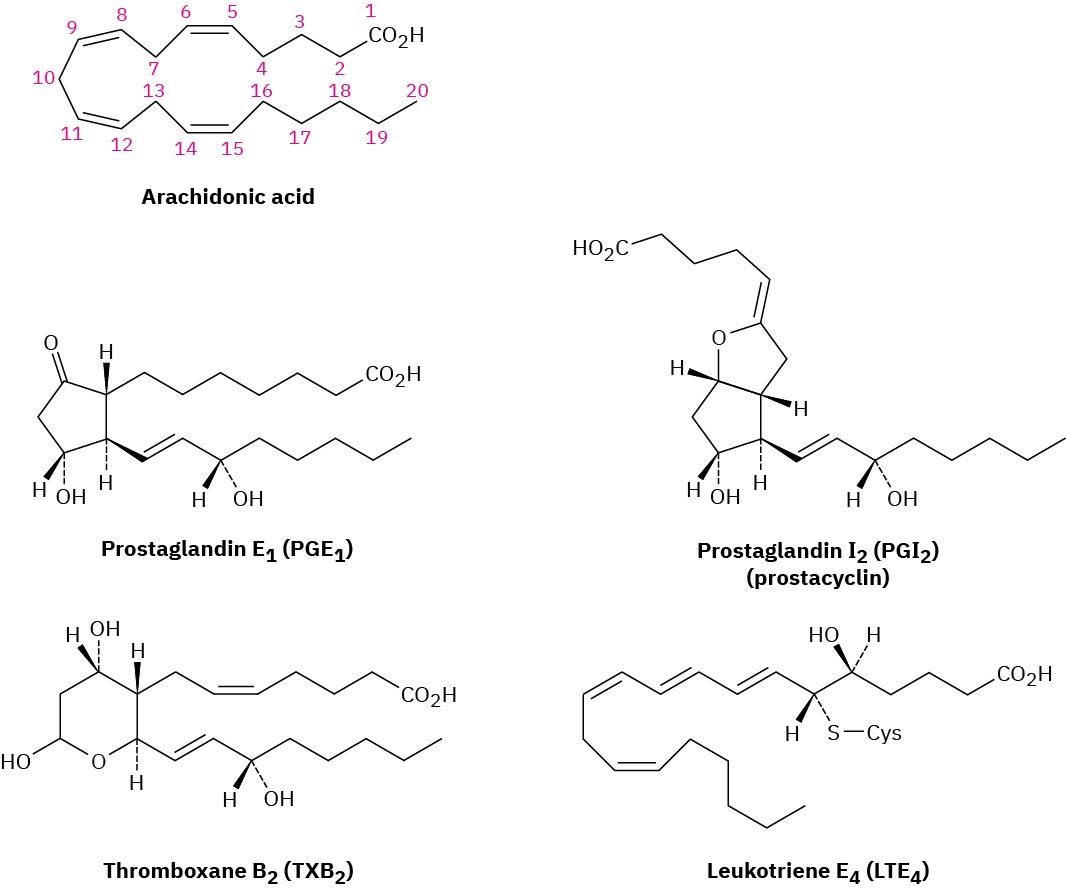
Figure 27.4 Structures of some representative eicosanoids. All are derived biologically from arachidonic acid.
Eicosanoids are named based on their ring system (PG, TX, or LT), substitution pattern, and number of double bonds. The various substitution patterns on the ring are indicated by letter as in Figure 27.5, and the number of double bonds is indicated by a subscript. Thus, PGE1 is a prostaglandin with the “E” substitution pattern and one double bond. The numbering of the atoms in the various eicosanoids is the same as in arachidonic acid, starting with the –CO2H carbon as C1, continuing around the ring, and ending with the – CH3 carbon at the other end of the chain as C20.
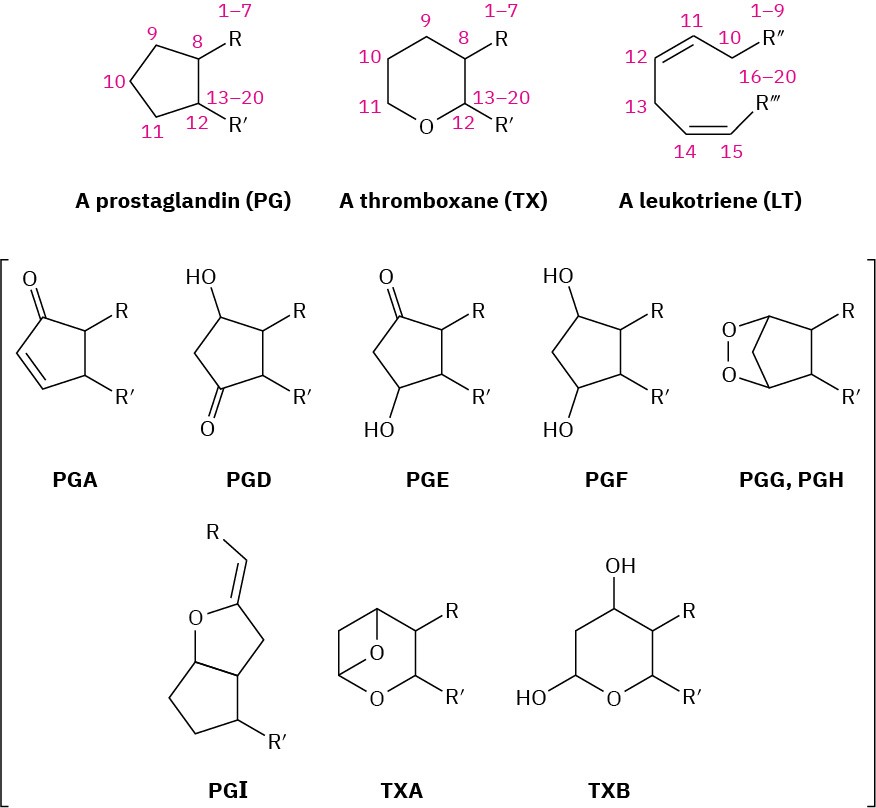
Figure 27.5The naming system for eicosanoids.
Eicosanoid biosynthesis begins with the conversion of arachidonic acid to PGH2, catalyzed by the multifunctional PGH synthase (PGHS), also called cyclooxygenase (COX). There are two distinct enzymes, PGHS-1 and PGHS-2 (or COX-1 and COX-2), both of which accomplish the same reaction but appear to function independently. COX-1 carries out the normal physiological production of prostaglandins, and COX-2 produces additional prostaglandin in response to arthritis or other inflammatory conditions. Vioxx, Bextra, and several other drugs selectively inhibit the COX-2 enzyme but cause potentially serious heart and gastrointestinal problems in weakened patients. (See the Chapter 15 Chemistry Matters.)
PGHS accomplishes two transformations, an initial reaction of arachidonic acid with O2 to yield PGG2 and a subsequent reduction of the hydroperoxide group (–OOH) to the alcohol PGH2. The sequence of steps involved in these transformations was shown in Figure 8.12.
Further processing of PGH2 leads to other eicosanoids. PGE2, for instance, arises by an isomerization of PGH2 catalyzed by PGE synthase (PGES) (Figure 27.6).
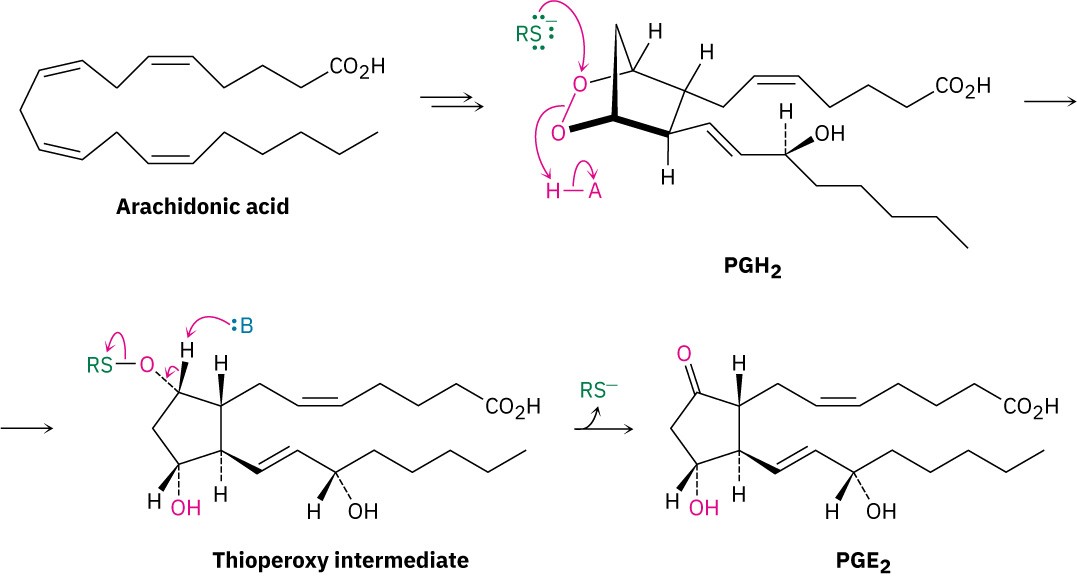
Figure 27.6Mechanism of the conversion of PGH2 into PGE2.
Problem 27-5
Assign R or S configuration to each chirality center in prostaglandin E2 (Figure 27.6), the most abundant and biologically potent of mammalian prostaglandins.

