Chemistry Matters — Degradable Polymers
The high chemical stability of many polymers is both a blessing and a curse. Heat resistance, wear resistance, and long life are valuable characteristics of clothing fibers, bicycle helmets, underground pipes, food wrappers, and many other items. Yet when these items outlive their usefulness, disposal becomes a problem.

Figure 31.8 What happens to the plastics that end up here? (credit: modification of work “King of the trash hill” by Alan Levine/Flickr, CC BY 2.0)
Recycling of unwanted polymers is the best solution, and six types of plastics in common use are frequently stamped with identifying codes assigned by the Society of the Plastics Industry Table 31.2. After being sorted by type, the items to be recycled are shredded into small chips, washed, dried, and melted for reuse. Soft-drink bottles, for instance, are made from recycled poly(ethylene terephthalate), trash bags are made from recycled low-density polyethylene, and garden furniture is made from recycled polypropylene and mixed plastics.
Table 31.2 Recyclable Plastics
|
Polymer |
Recycling code |
Use |
|
Poly(ethylene terephthalate) |
1—PET |
Soft-drink bottles |
|
High-density polyethylene |
2—HDPE |
Bottles |
|
Recycling code |
Use |
|
|
Poly(vinyl chloride) |
3—V |
Floor mats |
|
Low-density polyethylene |
4—LDPE |
Grocery bags |
|
Polypropylene |
5—PP |
Furniture |
|
Polystyrene |
6—PS |
Molded articles |
|
Mixed plastics |
7 |
Benches, plastic lumber |
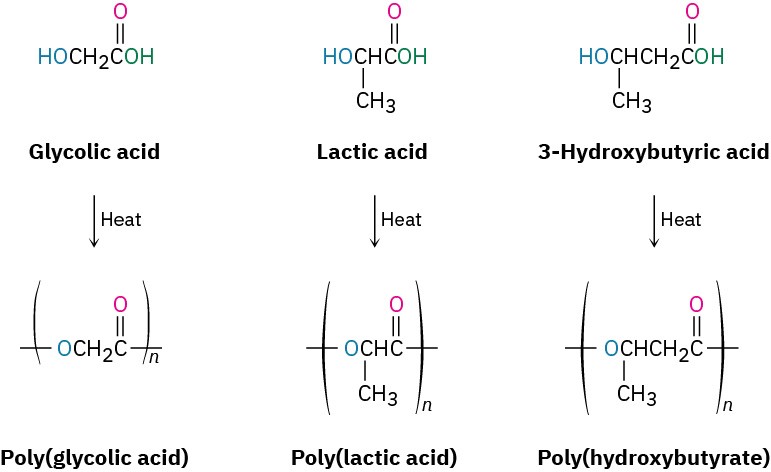
Frequently, however, plastics are simply thrown away rather than recycled, and much work has therefore been carried out on developing biodegradable polymers, which can be broken down by soil microorganisms. Among the most common biodegradable polymers are polyglycolic acid (PGA), polylactic acid (PLA), and polyhydroxybutyrate (PHB). All are polyesters and are therefore susceptible to hydrolysis of their ester links. Copolymers of PGA with PLA have found a particularly wide range of uses. A 90/10 copolymer of polyglycolic acid with polylactic acid is used to make absorbable sutures that are degraded and absorbed by the body within 90 days after surgery.
Biodegradation is important, but even better than the breakdown of a small group of specialized polymers would be the development of a generalized method of breakdown that could be used on any polymer, including even hydrocarbon polymers such as polyethylene and polypropylene. Such a method has recently been reported by chemists at the University of Delaware, using hydrogen at 200 °C and 2 atm pressure in the presence of an organozirconium catalyst to turn polyethylene into simple hydrocarbons. The method uses a large amount of hydrogen at present, but it is an impressive start.

