5.12 Chirality in Nature and Chiral Environments
Although the different enantiomers of a chiral molecule have the same physical properties, they usually have different biological properties. For example, a change in chirality can affect the biological properties of many drugs, such as fluoxetine, a heavily prescribed medication sold under the trade name Prozac. Racemic fluoxetine is an effective antidepressant but has no activity against migraine. The pure S enantiomer, however, works remarkably well in preventing migraine. Other examples of how chirality affects biological properties are given in the Chapter 5 Chemistry Matters at the end of this chapter.
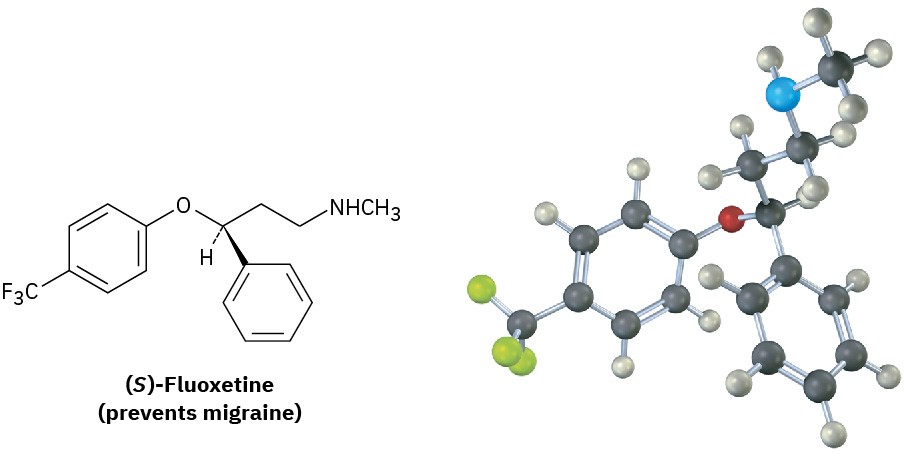
Why do different enantiomers have different biological properties? To have a biological effect, a substance typically must fit into an appropriate receptor that has a complementary shape. But because biological receptors are chiral, only one enantiomer of a chiral substrate can fit, just as only a right hand can fit into a right-handed glove. The mirror-image enantiomer will be a misfit, like a left hand in a right-handed glove. A representation of the interaction between a chiral molecule and a chiral biological receptor is shown in Figure 5.16: one enantiomer fits the receptor perfectly, but the other does not.
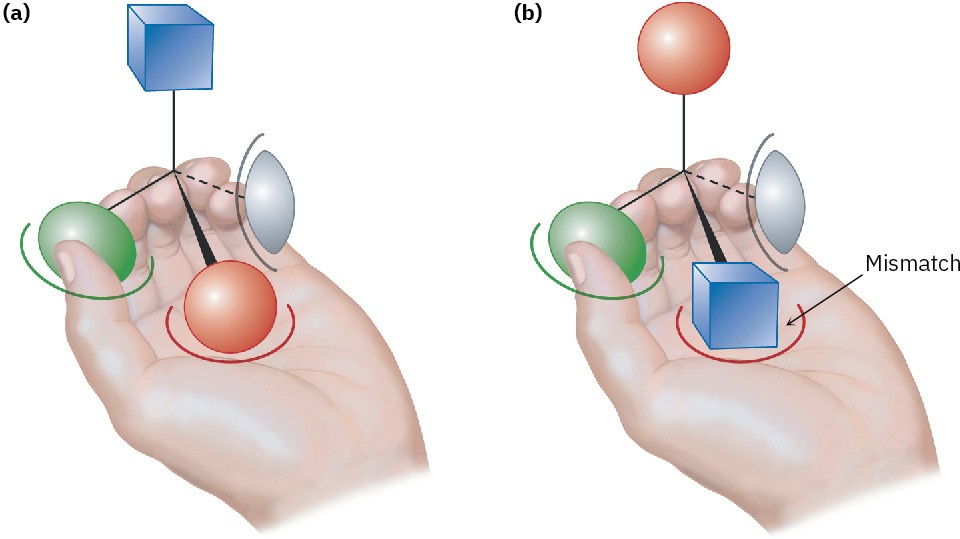
Figure 5.16 Interaction of a chiral object with a chiral receptor. A left hand interacts with a chiral object, much as a biological receptor interacts with a chiral molecule. (a) One enantiomer fits into the hand perfectly: green thumb, red palm, and gray pinkie finger, with the blue substituent exposed. (b) The other enantiomer, however, can’t fit into the hand. When the green thumb and gray pinkie finger interact appropriately, the palm holds a blue substituent rather than a red one, with the red substituent exposed.
The hand-in-glove fit of a chiral substrate into a chiral receptor is relatively straightforward, but it’s less obvious how a prochiral substrate can undergo a selective reaction. Take the reaction of ethanol with NAD+ catalyzed by yeast alcohol dehydrogenase. As we saw at the end of Section 5.11, this reaction occurs with exclusive removal of the pro- R hydrogen from ethanol and with addition only to the Re face of the NAD+ carbon.
We can understand this result by imagining that the chiral enzyme receptor again has three binding sites, as in Figure 5.16. When green and gray substituents of a prochiral substrate are held appropriately, however, only one of the two red substituents—say, the pro-S one—is also held while the other, pro-R, substituent is exposed for reaction.
We describe the situation by saying that the receptor provides a chiral environment for the substrate. In the absence of a chiral environment, the two red substituents are chemically identical, but in the presence of a chiral environment, they are chemically distinctive (Figure 5.17a). The situation is similar to what happens when you pick up a coffee mug. By itself, the mug has a plane of symmetry and is achiral. When you pick up the mug, however, your hand provides a chiral environment so that one side becomes much more accessible and easier to drink from than the other (Figure 5.17b).
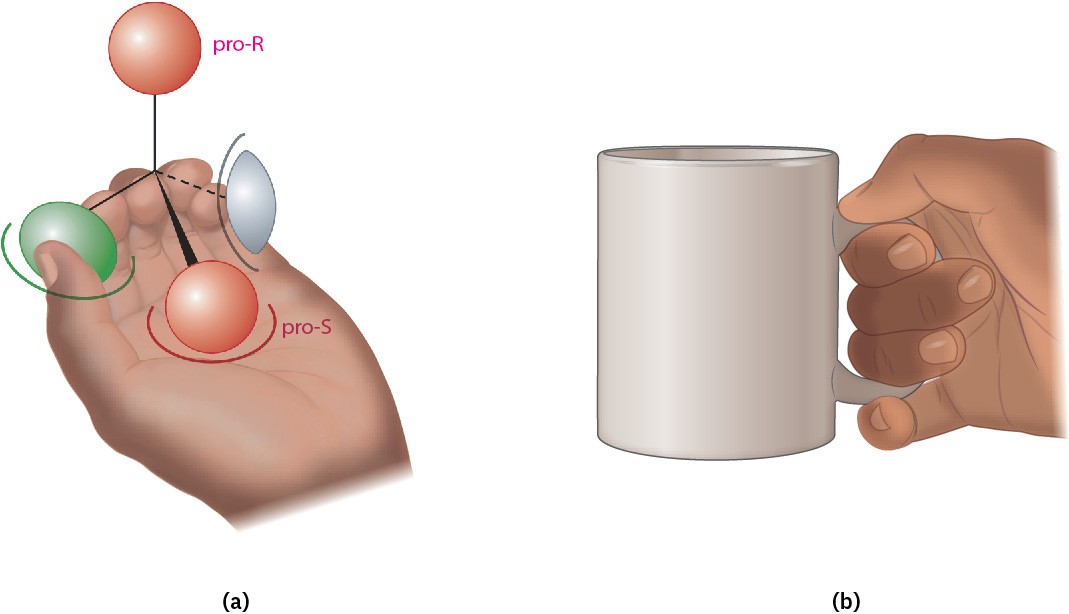
Figure 5.17 (a) When a prochiral molecule is held in a chiral environment, the two seemingly identical substituents are distinguishable. (b) Similarly, when an achiral coffee mug is held in the chiral environment of your hand, it’s much easier to drink from one side than the other because the two sides of the mug are now distinguishable.
Additional Problems 5 • Additional Problems 5 • Additional Problems Visualizing Chemistry Problem 5-26
Which of the following structures are identical? (Green = Cl.)
(a) 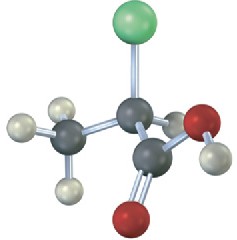 (b)
(b) 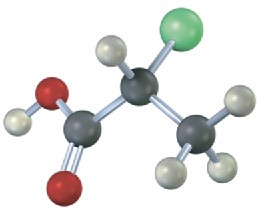 (c)
(c) 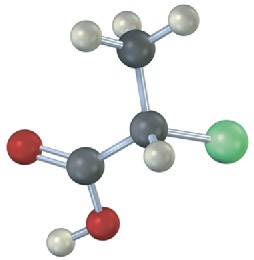 (d)
(d) 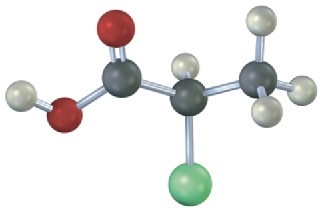
Problem 5-27
Assign R or S configurations to the chirality centers in the following molecules (blue = N): (a)
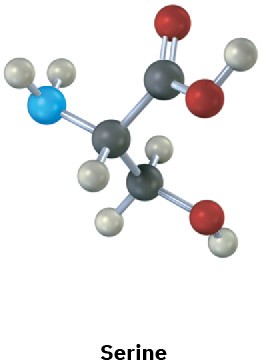
(b)
Problem 5-28
Which, if any, of the following structures represent meso compounds? (Blue = N, green = Cl.)
(a)
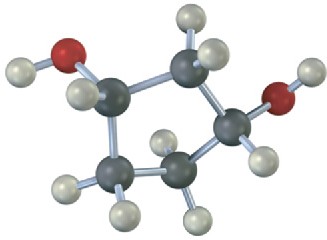
(b)
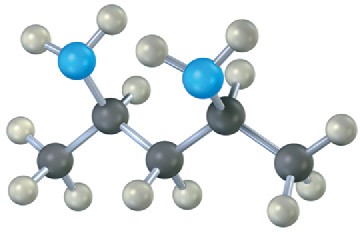
(c)
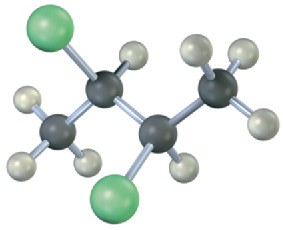
Problem 5-29
Assign R or S configuration to each chirality center in pseudoephedrine, an over-the- counter decongestant found in cold remedies (blue = N).
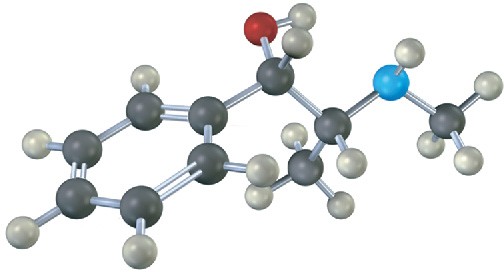
Problem 5-30
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
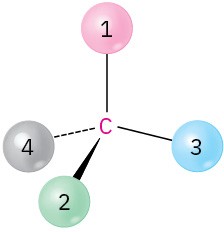
(b)
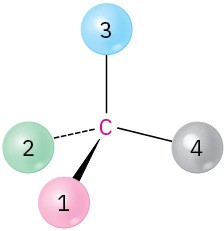
(c)
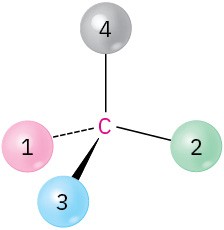
Chirality and Optical Activity
Problem 5-31
Which of the following objects are chiral?
(a) A basketball(b) A fork(c) A wine glass(d) A golf club(e) A spiral staircase
(f) A snowflake Problem 5-32
Which of the following compounds are chiral? Draw them, and label the chirality centers. (a)
2,4-Dimethylheptane (b)
5-Ethyl-3,3-dimethylheptane (c)
cis-1,4-Dichlorocyclohexane Problem 5-33
Draw chiral molecules that meet the following descriptions: (a)
A chloroalkane, C5H11Cl (b)
An alcohol, C6H14O (c)
An alkene, C6H12 (d)
An alkane, C8H18 Problem 5-34
Eight alcohols have the formula C5H12O. Draw them. Which are chiral? Problem 5-35
Draw compounds that fit the following descriptions: (a)
A chiral alcohol with four carbons (b)
A chiral carboxylic acid with the formula C5H10O2 (c)
A compound with two chirality centers (d)
A chiral aldehyde with the formula C3H5BrO Problem 5-36
Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How many chirality centers does erythronolide B have? Identify them.
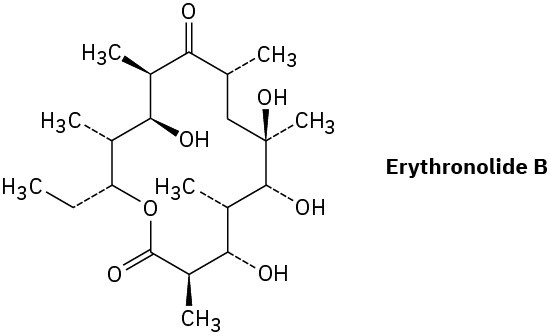
Assigning Configuration to Chirality Centers
Problem 5-37
Which of the following pairs of structures represent the same enantiomer, and which represent different enantiomers?
(a)
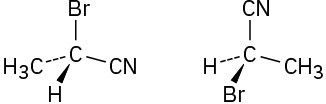
(b)
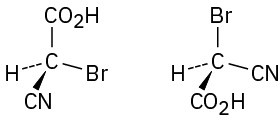
(c)
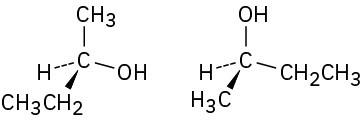
(d)
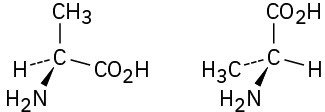
Problem 5-38
What is the relationship between the specific rotations of (2R,3R)-dichloropentane and (2S,3S)-dichloropentane? Between (2R,3S)-dichloropentane and (2R,3R)-dichloropentane?
Problem 5-39
What is the stereochemical configuration of the enantiomer of (2S,4R)-2,4-octanediol? Problem 5-40
What are the stereochemical configurations of the two diastereomers of (2S,4R)-2,4- octanediol? (A diol is a compound with two –OH groups.)
Problem 5-41
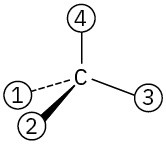
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
(b)
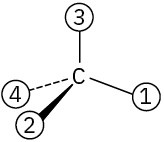
(c)
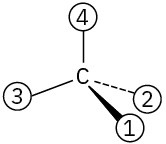
Problem 5-42
Assign Cahn–Ingold–Prelog rankings to the following sets of substituents: (a)
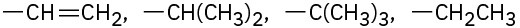
(b)
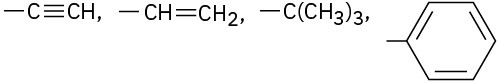
(c)
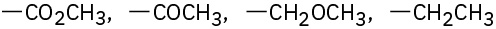
(d)
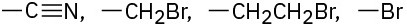
Problem 5-43
Assign R or S configurations to each chirality center in the following molecules: (a)
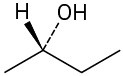
(b)
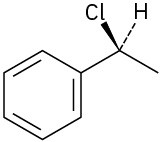
(c)
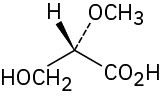
Problem 5-44
Assign R or S configuration to each chirality center in the following molecules: (a)
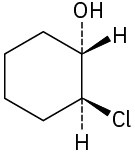
(b)
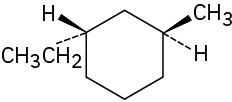
(c)
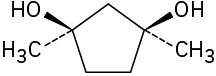
Problem 5-45
Assign R or S configuration to each chirality center in the following biological molecules: (a)
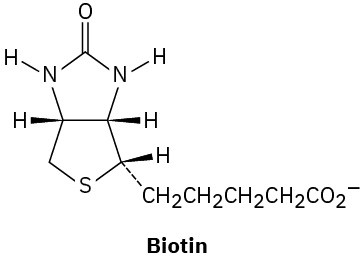
(b)
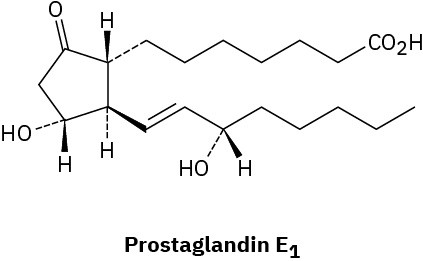
Problem 5-46
Draw tetrahedral representations of the following molecules: (a)
(S)-2-Chlorobutane (b)
(R)-3-Chloro-1-pentene [H2C=CHCH(Cl)CH2CH3] Problem 5-47
Assign R or S configuration to each chirality center in the following molecules: (a)
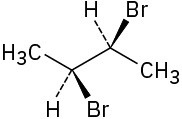
(b)
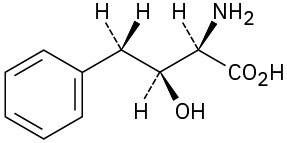
Problem 5-48
Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C).
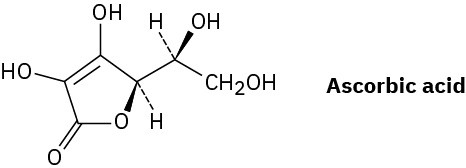
Problem 5-49
Assign R or S stereochemistry to the chirality centers in the following Newman projections: (a)
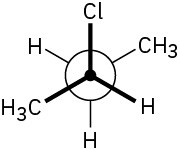
(b)
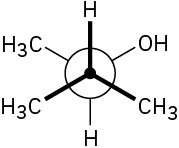
Problem 5-50
Xylose is a common sugar found in many types of wood, including maple and cherry. Because it is much less prone to cause tooth decay than sucrose, xylose has been used in candy and chewing gum. Assign R or S configurations to the chirality centers in xylose.
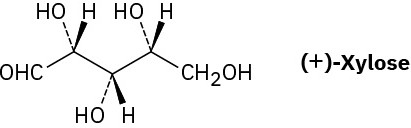
Meso Compounds
Problem 5-51
Draw examples of the following:
(a)
A meso compound with the formula C8H18 (b)
A meso compound with the formula C9H20 (c)
A compound with two chirality centers, one R and the other S
Problem 5-52
Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each:
(a)
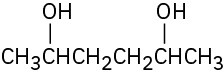
(b)
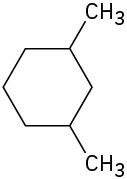
(c)
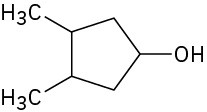
Problem 5-53
Draw the structure of a meso compound that has five carbons and three chirality centers. Problem 5-54
Ribose, an essential part of ribonucleic acid (RNA), has the following structure:
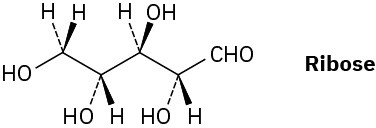
(a)
How many chirality centers does ribose have? Identify them. (b)
How many stereoisomers of ribose are there? (c)
Draw the structure of the enantiomer of ribose. (d)
Draw the structure of a diastereomer of ribose. Problem 5-55
On reaction with hydrogen gas in the presence of a platinum catalyst, ribose (Problem 5- 54) is converted into ribitol. Is ribitol optically active or inactive? Explain.
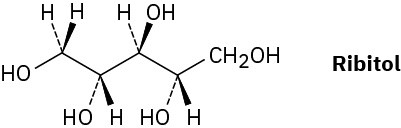
Prochirality
Problem 5-56
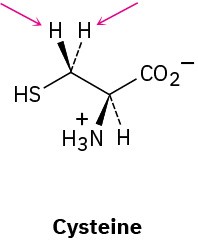
Identify the indicated hydrogens in the following molecules as pro-R or pro-S: (a)
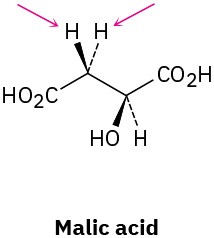
(b)
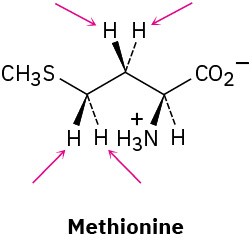
(c)
Problem 5-57
Identify the indicated faces in the following molecules as Re or Si: (a)
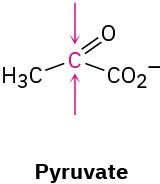
(b)
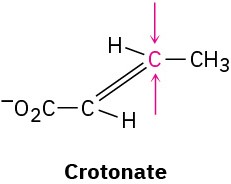
Problem 5-58
One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. The reaction occurs by addition of –OH to the Si face at C3, followed by protonation at C2, also from the Si face. Draw the product of the reaction, showing the stereochemistry of each step.
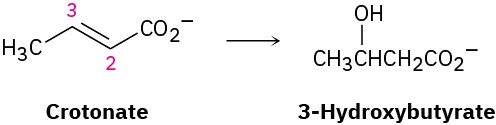
Problem 5-59
The dehydration of citrate to yield cis-aconitate, a step in the citric acid cycle, involves the pro-R “arm” of citrate rather than the pro-S arm. Which of the following two products is formed?
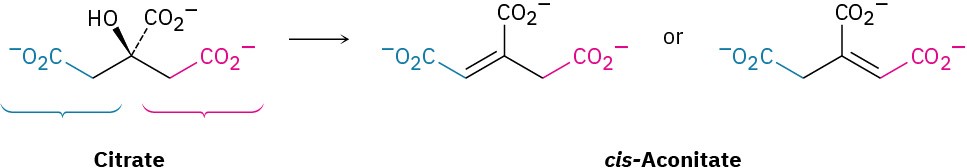
Problem 5-60
The first step in the metabolism of glycerol, formed by digestion of fats, is phosphorylation of the pro-R –CH2OH group by reaction with adenosine triphosphate (ATP) to give the corresponding glycerol phosphate plus adenosine diphosphate (ADP). Show the stereochemistry of the product.
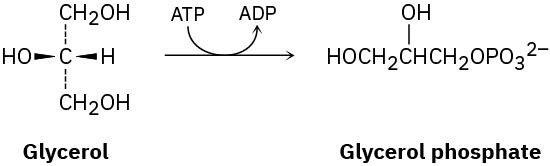
Problem 5-61
One of the steps in fatty-acid biosynthesis is the dehydration of (R)-3-hydroxybutyryl ACP to give trans-crotonyl ACP. Does the reaction remove the pro-R or the pro-S hydrogen from C2?
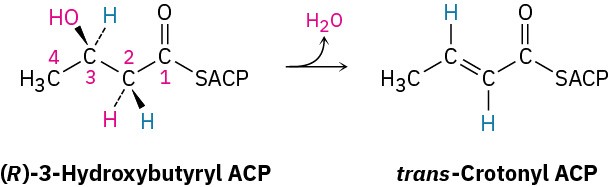
General Problems
Problem 5-62
Draw all possible stereoisomers of 1,2-cyclobutanedicarboxylic acid, and indicate the interrelationships. Which, if any, are optically active? Do the same for 1,3- cyclobutanedicarboxylic acid.
Problem 5-63
Draw tetrahedral representations of the two enantiomers of the amino acid cysteine, HSCH2CH(NH2)CO2H, and identify each as R or S.
Problem 5-64
The naturally occurring form of the amino acid cysteine (Problem 5-63) has the R configuration at its chirality center. On treatment with a mild oxidizing agent, two cysteines join to give cystine, a disulfide. Assuming that the chirality center is not affected by the reaction, is cystine optically active? Explain.
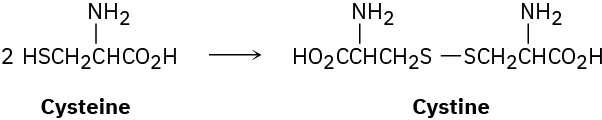
Problem 5-65
Draw tetrahedral representations of the following molecules: (a)
The 2S,3R enantiomer of 2,3-dibromopentane (b)
The meso form of 3,5-heptanediol Problem 5-66
Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States.
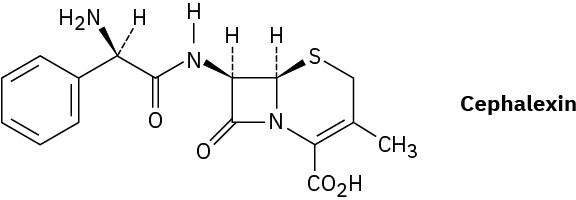
Problem 5-67
Chloramphenicol, a powerful antibiotic isolated in 1947 from the Streptomyces venezuelae bacterium, is active against a broad spectrum of bacterial infections and is particularly valuable against typhoid fever. Assign R or S configurations to the chirality centers in chloramphenicol.
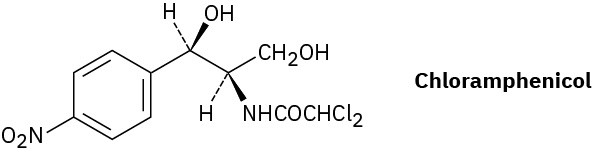
Problem 5-68
Allenes are compounds with adjacent carbon–carbon double bonds. Many allenes are chiral, even though they don’t contain chirality centers. Mycomycin, for example, a naturally occurring antibiotic isolated from the bacterium Nocardia acidophilus, is chiral and has [α]D
= −130. Explain why mycomycin is chiral.
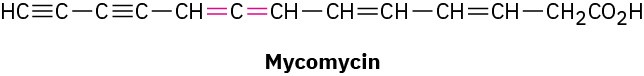
Problem 5-69
Long before chiral allenes were known (Problem 5-68), the resolution of 4- methylcyclohexylideneacetic acid into two enantiomers had been carried out. Why is it chiral? What geometric similarity does it have to allenes?
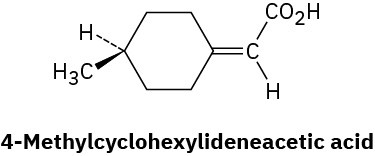
Problem 5-70
(S)-1-Chloro-2-methylbutane undergoes light-induced reaction with Cl2 to yield a mixture of products, among which are 1,4-dichloro-2-methylbutane and 1,2-dichloro-2- methylbutane.
(a)
Write the reaction, showing the correct stereochemistry of the reactant. (b)
One of the two products is optically active, but the other is optically inactive. Which is which?
Problem 5-71
How many stereoisomers of 2,4-dibromo-3-chloropentane are there? Draw them, and indicate which are optically active.
Problem 5-72
Draw both cis– and trans-1,4-dimethylcyclohexane in their more stable chair conformations.
(a)
How many stereoisomers are there of cis-1,4-dimethylcyclohexane, and how many of trans– 1,4-dimethylcyclohexane?
(b)
Are any of the structures chiral? (c)
What are the stereochemical relationships among the various stereoisomers of 1,4- dimethylcyclohexane?
Problem 5-73
Draw both cis– and trans-1,3-dimethylcyclohexane in their more stable chair conformations.
(a)
How many stereoisomers are there of cis-1,3-dimethylcyclohexane, and how many of trans– 1,3-dimethylcyclohexane?
(b)
Are any of the structures chiral? (c)
What are the stereochemical relationships among the various stereoisomers of 1,3- dimethylcyclohexane?
Problem 5-74
cis-1,2-Dimethylcyclohexane is optically inactive even though it has two chirality centers. Explain.
Problem 5-75
We’ll see in Chapter 11 that alkyl halides react with hydrosulfide ion (HS−) to give a product whose stereochemistry is inverted from that of the reactant.
Draw the reaction of (S)-2-bromobutane with HS− ion to yield 2-butanethiol, CH3CH2CH(SH)CH3. Is the stereochemistry of the product R or S?
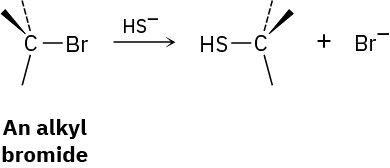
Problem 5-76
Ketones react with sodium acetylide (the sodium salt of acetylene, Na+– : C≡CH) to give alcohols. For example, the reaction of sodium acetylide with 2-butanone yields 3-methyl-1- pentyn-3-ol:
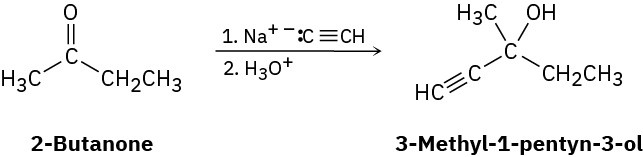
(a)
Is the product chiral? (b)
Assuming that the reaction takes place with equal likelihood from both Re and Si faces of the carbonyl group, is the product optically active? Explain.
Problem 5-77
Imagine that a reaction similar to that in Problem 5-76 is carried out between sodium acetylide and (R)-2-phenylpropanal to yield 4-phenyl-1-pentyn-3-ol:
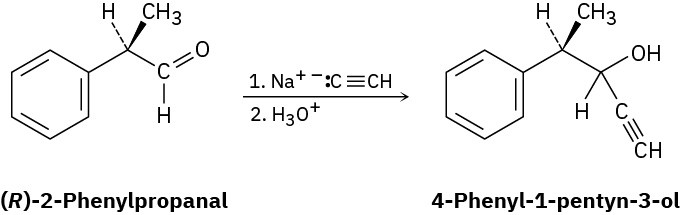
(a)
Is the product chiral? (b)
Draw both major and minor reaction products, assuming that the reaction takes place preferentially from the Re face of the carbonyl group. Is the product mixture optically active? Explain.

