1.2 Atomic Structure: Orbitals
How are the electrons distributed in an atom? You might recall from your general chemistry course that, according to the quantum mechanical model, the behavior of a specific electron in an atom can be described by a mathematical expression called a wave equation—the same type of expression used to describe the motion of waves in a fluid. The solution to a wave equation is called a wave function, or orbital, and is denoted by the lowercase Greek letter psi (ψ).
When the square of the wave function, ψ2, is plotted in three-dimensional space, an orbital describes the volume of space around a nucleus that an electron is most likely to occupy. You might therefore think of an orbital as looking like a photograph of the electron taken at a slow shutter speed. In such a photo, the orbital would appear as a blurry cloud, indicating the region of space where the electron has been. This electron cloud doesn’t have a sharp boundary, but for practical purposes we can set the limits by saying that an orbital represents the space where an electron spends 90% to 95% of its time.
What do orbitals look like? There are four different kinds of orbitals, denoted s, p, d, and f, each with a different shape. Of the four, we’ll be concerned primarily with s and p orbitals because these are the most common in organic and biological chemistry. An s orbital has a spherical shape, with the nucleus at its center; a p orbital has a dumbbell shape with two parts, or lobes; and four of the five d orbitals have a cloverleaf shape with four lobes, as shown in Figure 1.4. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle.
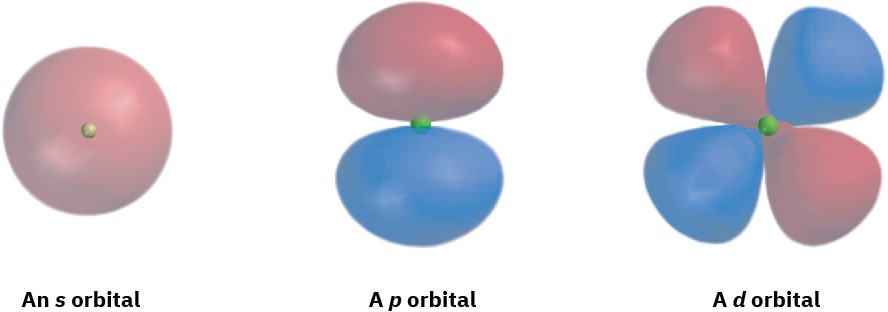
Figure 1.4 Representations of s, p, and d orbitals. An s orbital is spherical, a p orbital is dumbbell-shaped, and four of the five d orbitals are cloverleaf-shaped. Different lobes of p orbitals are often drawn for convenience as teardrops, but their actual shape is more like that of a doorknob, as indicated.
The orbitals in an atom are organized into different layers around the nucleus called electron shells, which are centered around the nucleus and have successively larger size and energy. Different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. The first shell contains only a single s orbital, denoted 1s, and thus holds only 2 electrons. The second shell contains one 2s
orbital and three 2p orbitals and thus holds a total of 8 electrons. The third shell contains a 3s orbital, three 3p orbitals, and five 3d orbitals, for a total capacity of 18 electrons. These orbital groupings and their energy levels are shown in Figure 1.5.
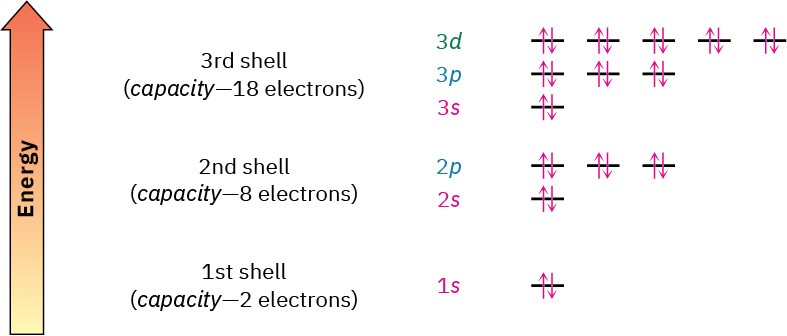
Figure 1.5 Energy levels of electrons in an atom. The first shell holds a maximum of 2 electrons in one 1s orbital; the second shell holds a maximum of 8 electrons in one 2s and three 2p orbitals; the third shell holds a maximum of 18 electrons in one 3s, three 3p, and 3d orbitals; and so on. The two electrons in each orbital are represented by five up and down arrows, ⇅. Although not shown, the energy level of the 4s orbital falls between 3p and 3d.
The three different p orbitals within a given shell are oriented in space along mutually perpendicular directions, denoted px, py, and pz. As shown in Figure 1.6, the two lobes of each p orbital are separated by a region of zero electron density called a node.
Furthermore, the two orbital regions separated by the node have different algebraic signs,
+ and −, in the wave function, as represented by the different colors in Figure 1.4 and Figure 1.6. As we’ll see in Section 1.11, these algebraic signs for different orbital lobes have important consequences with respect to chemical bonding and chemical reactivity.
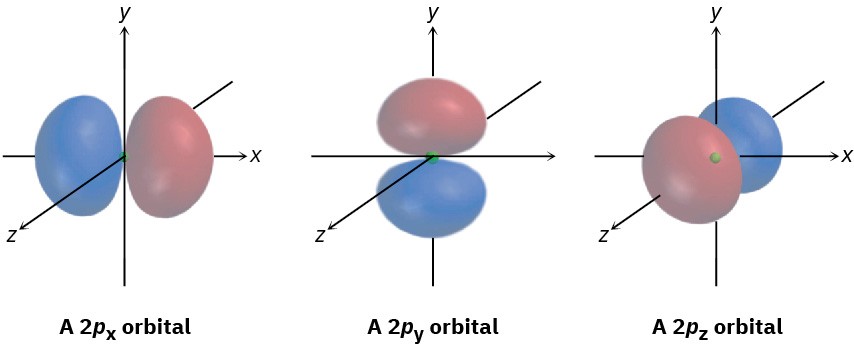
Figure 1.6 Shapes of the 2p orbitals. Each of the three mutually perpendicular, dumbbell-shaped orbitals has two lobes separated by a node. The two lobes have different algebraic signs in the corresponding wave function, as indicated by the different colors.

