Additional Problems 12
Visualizing Chemistry
Problem 12-12
Where in the IR spectrum would you expect each of the following molecules to absorb?
(a)
(b)
(c)
Problem 12-13
Show the structures of the fragments you would expect in the mass spectra of the following molecules:
(a)
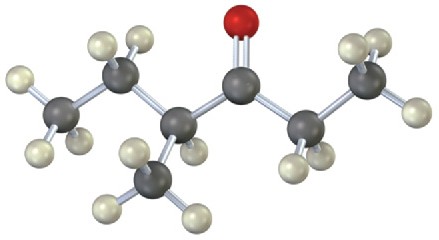
(b)
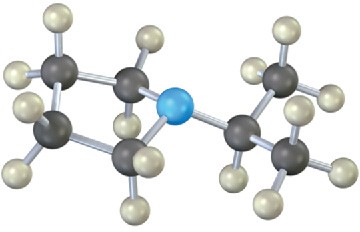
Mass Spectrometry
Problem 12-14
Propose structures for compounds that fit the following mass-spectral data:
(a) A hydrocarbon with M+ = 132
(b) A hydrocarbon with M+ = 166
(c) A hydrocarbon with M+ = 84
Problem 12-15
Write molecular formulas for compounds that show the following molecular ions in their high-resolution mass spectra, assuming that C, H, N, and O might be present. The exact atomic masses are: 1.007 83 (1H), 12.000 00 (12C), 14.003 07 (14N), 15.994 91 (16O).
(a) M+ = 98.0844
(b) M+ = 123.0320
Problem 12-16
Camphor, a saturated monoketone from the Asian camphor tree, is used among other things as a moth repellent and as a constituent of embalming fluid. If camphor has M+ = 152.1201 by high-resolution mass spectrometry, what is its molecular formula? How many rings does camphor have?
Problem 12-17
The nitrogen rule of mass spectrometry says that a compound containing an odd number of nitrogens has an odd-numbered molecular ion. Conversely, a compound containing an even number of nitrogens has an even-numbered M+ peak. Explain.
Problem 12-18
In light of the nitrogen rule mentioned in Problem 12-17, what is the molecular formula of pyridine, M+ = 79?
Problem 12-19
Nicotine is a diamino compound isolated from dried tobacco leaves. Nicotine has two rings and M+ = 162.1157 by high-resolution mass spectrometry. Give a molecular formula for nicotine, and calculate the number of double bonds.
Problem 12-20
The hormone cortisone contains C, H, and O, and shows a molecular ion at M+ = 360.1937 by high-resolution mass spectrometry. What is the molecular formula of cortisone? (The degree of unsaturation for cortisone is 8.)
Problem 12-21
Halogenated compounds are particularly easy to identify by their mass spectra because both chlorine and bromine occur naturally as mixtures of two abundant isotopes. Recall that chlorine occurs as 35Cl (75.8%) and 37Cl (24.2%); and bromine occurs as 79Br (50.7%) and 81Br (49.3%). At what masses do the molecular ions occur for the following formulas? What are the relative percentages of each molecular ion?
(a) Bromomethane, CH3Br
(b) Chlorohexane, C6H13Cl
Problem 12-22
By knowing the natural abundances of minor isotopes, it’s possible to calculate the relative heights of M+ and M + 1 peaks. If 13C has a natural abundance of 1.10%, what are the relative heights of the M+ and M + 1 peaks in the mass spectrum of benzene, C6H6?
Problem 12-23
Propose structures for compounds that fit the following data:
(a) A ketone with M+ = 86 and fragments at m/z = 71 and m/z = 43
(b) An alcohol with M+ = 88 and fragments at m/z = 73, m/z = 70, and m/z = 59
Problem 12-24
Methylpentane (C6H14) has the mass spectrum shown. Which peak represents M+? Which is the base peak? Propose structures for fragment ions of m/z = 71, 57, 43, and 29. Why does the base peak have the mass it does?
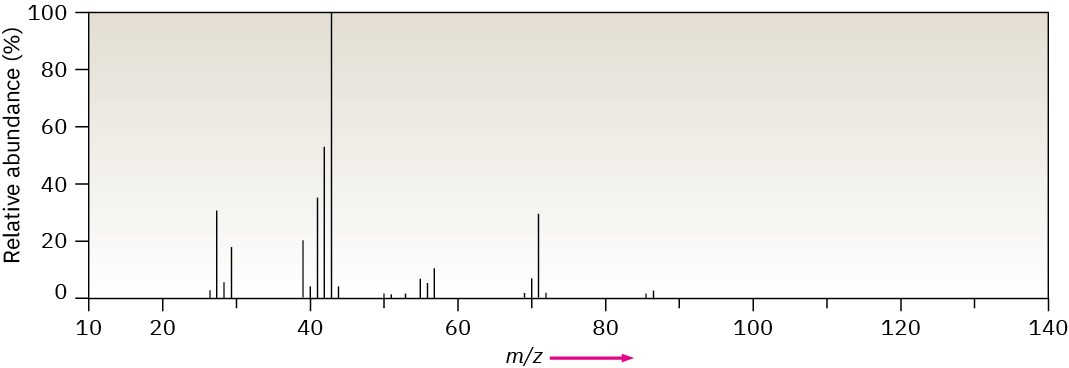
Problem 12-25
Assume that you are in a laboratory carrying out the catalytic hydrogenation of cyclohexene to cyclohexane. How could you use a mass spectrometer to determine when the reaction is finished?
Problem 12-26
What fragments might you expect in the mass spectra of the following compounds?
(a)
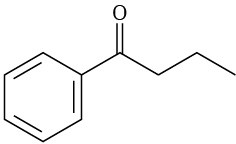
(b)
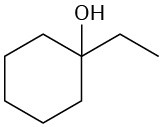
(c)
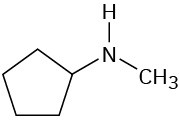
Infrared Spectroscopy
Problem 12-27
How might you use IR spectroscopy to distinguish among the three isomers 1-butyne, 1,3- butadiene, and 2-butyne?
Problem 12-28
Would you expect two enantiomers such as (R)-2-bromobutane and (S)-2-bromobutane to have identical or different IR spectra? Explain.
Problem 12-29
Would you expect two diastereomers such as meso-2,3-dibromobutane and (2R,3R)- dibromobutane to have identical or different IR spectra? Explain.
Problem 12-30
Propose structures for compounds that meet the following descriptions:
(a) C5H8, with IR absorptions at 3300 and 2150 cm–1
(b) C4H8O, with a strong IR absorption at 3400 cm–1
(c) C4H8O, with a strong IR absorption at 1715 cm–1
(d) C8H10, with IR absorptions at 1600 and 1500 cm–1
Problem 12-31
How could you use infrared spectroscopy to distinguish between the following pairs of isomers?
(a) HC≡CCH2NH2 and CH3CH2C≡N
(b) CH3COCH3 and CH3CH2CHO
Problem 12-32
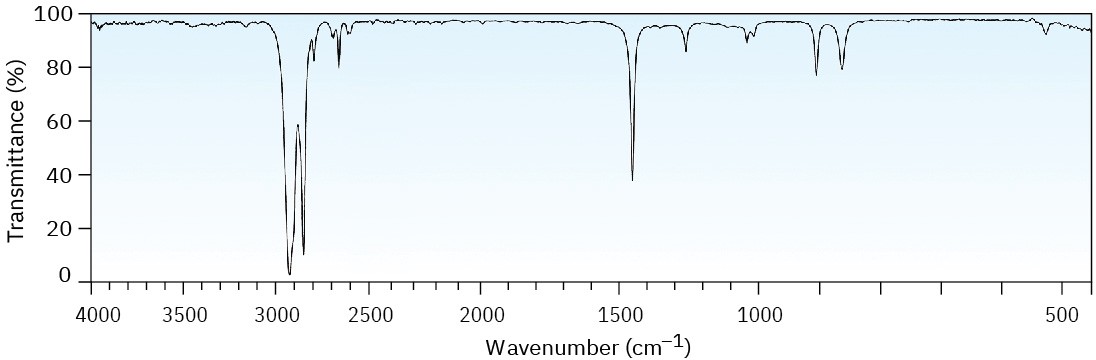
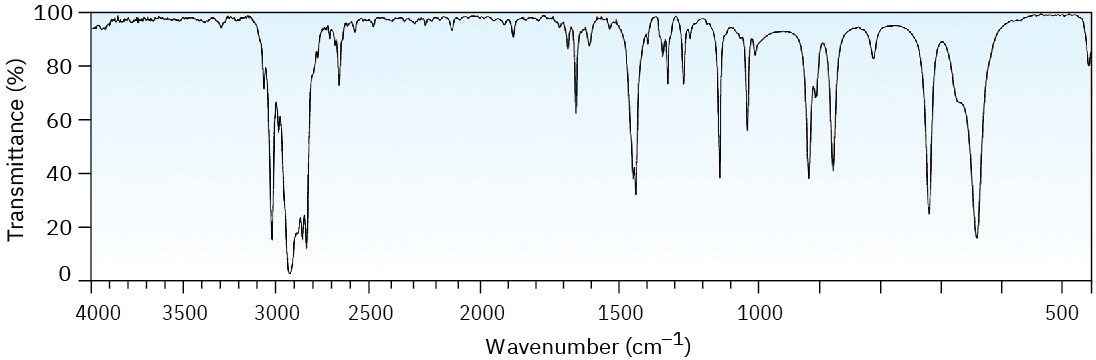
Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum of cyclohexene. Identify them, and explain your answer.
(a)
(b)
Problem 12-33
At what approximate positions might the following compounds show IR absorptions?
(a)
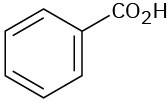
(b)
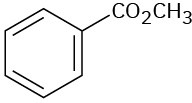
(c)
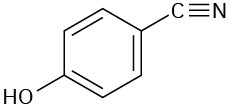
(d)
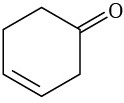
(e)
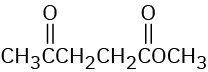
Problem 12-34
How would you use infrared spectroscopy to distinguish between the following pairs of constitutional isomers?
(a)
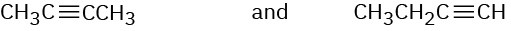
(b)

(c)
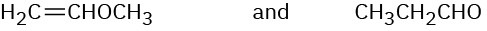
Problem 12-35
At what approximate positions might the following compounds show IR absorptions?
(a)
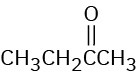
(b)
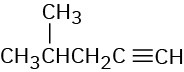
(c)
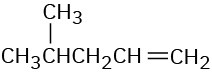
(d)
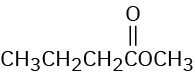
(e)
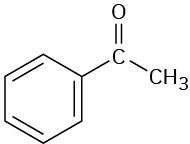
(f)
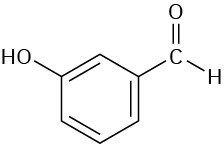
Problem 12-36
Assume that you are carrying out the dehydration of 1-methylcyclohexanol to yield 1- methylcyclohexene. How could you use infrared spectroscopy to determine when the reaction is complete?
Problem 12-37
Assume that you are carrying out the base-induced dehydrobromination of 3-bromo-3- methylpentane (Section 11.7) to yield an alkene. How could you use IR spectroscopy to tell which of three possible elimination products is formed, if one includes E/Z isomers?
General Problems
Problem 12-38
Which is stronger, the C=O bond in an ester (1735 cm–1) or the C=O bond in a saturated ketone (1715 cm–1)? Explain.
Problem 12-39
Carvone is an unsaturated ketone responsible for the odor of spearmint. If carvone has M+
= 150 in its mass spectrum and contains three double bonds and one ring, what is its molecular formula?
Problem 12-40
Carvone (Problem 12-39) has an intense infrared absorption at 1690 cm–1. What kind of ketone does carvone contain?
Problem 12-41
The mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can.
(a)
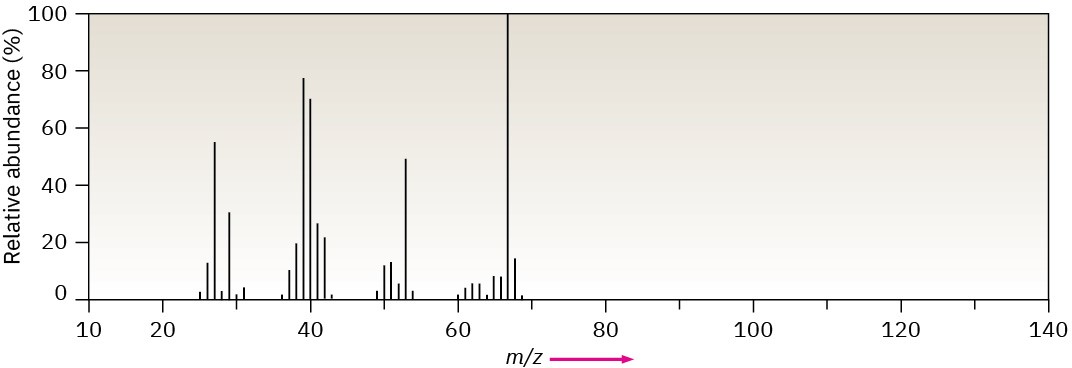
(b)
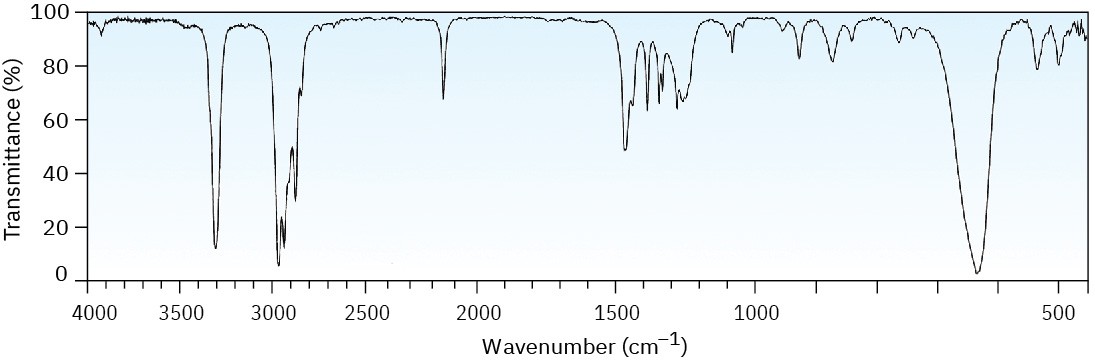
Problem 12-42
The mass spectrum (a) and the infrared spectrum (b) of another unknown hydrocarbon are shown. Propose as many structures as you can.
(a)
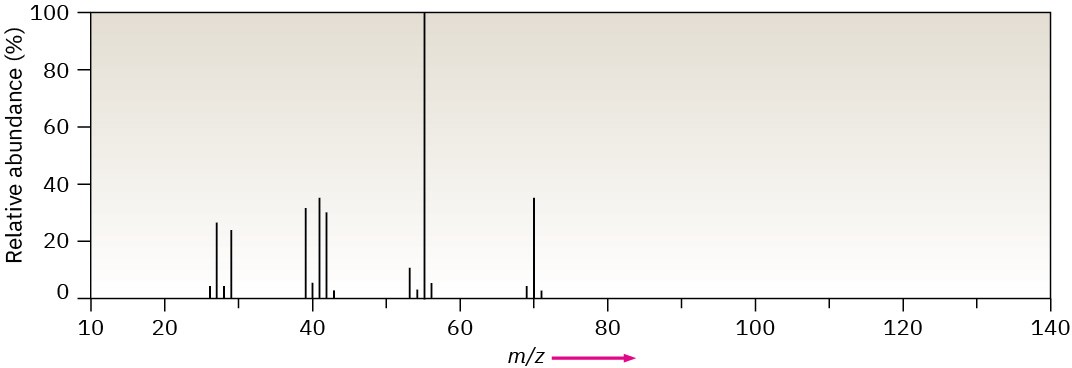
(b)
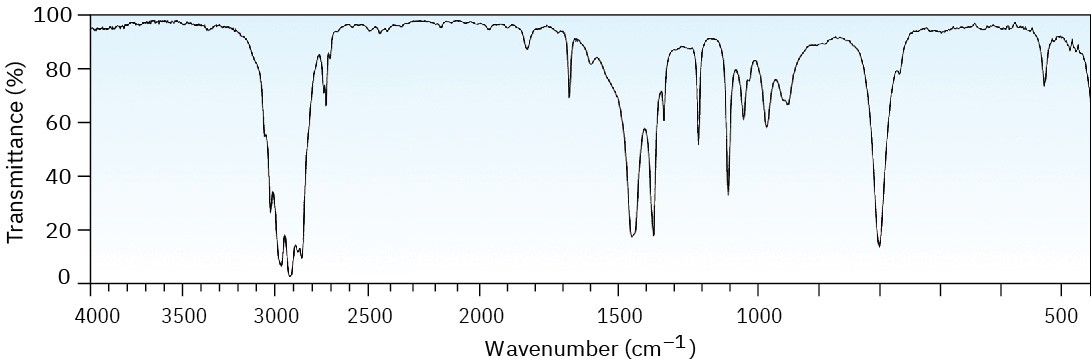
Problem 12-43
Propose structures for compounds that meet the following descriptions:
(a) An optically active compound C5H10O with an IR absorption at 1730 cm–1
(b) A non-optically active compound C5H9N with an IR absorption at 2215 cm–1
Problem 12-44
4-Methyl-2-pentanone and 3-methylpentanal are isomers. Explain how you could tell them apart, both by mass spectrometry and by infrared spectroscopy.

Problem 12-45
Grignard reagents (alkylmagnesium halides) undergo a general and very useful reaction with ketones. Methylmagnesium bromide, for example, reacts with cyclohexanone to yield a product with the formula C7H14O. What is the structure of this product if it has an IR absorption at 3400 cm–1?
Problem 12-46
Ketones undergo a reduction when treated with sodium borohydride, NaBH4. What is the structure of the compound produced by reaction of 2-butanone with NaBH4 if it has an IR absorption at 3400 cm–1 and M+ = 74 in the mass spectrum?
Problem 12-47
Nitriles, R–C≡N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propanenitrile, CH3CH2C≡N, if it has IR absorptions from 2500–3100 cm–1 and at 1710 cm–1, and has M+ = 74?
Problem 12-48
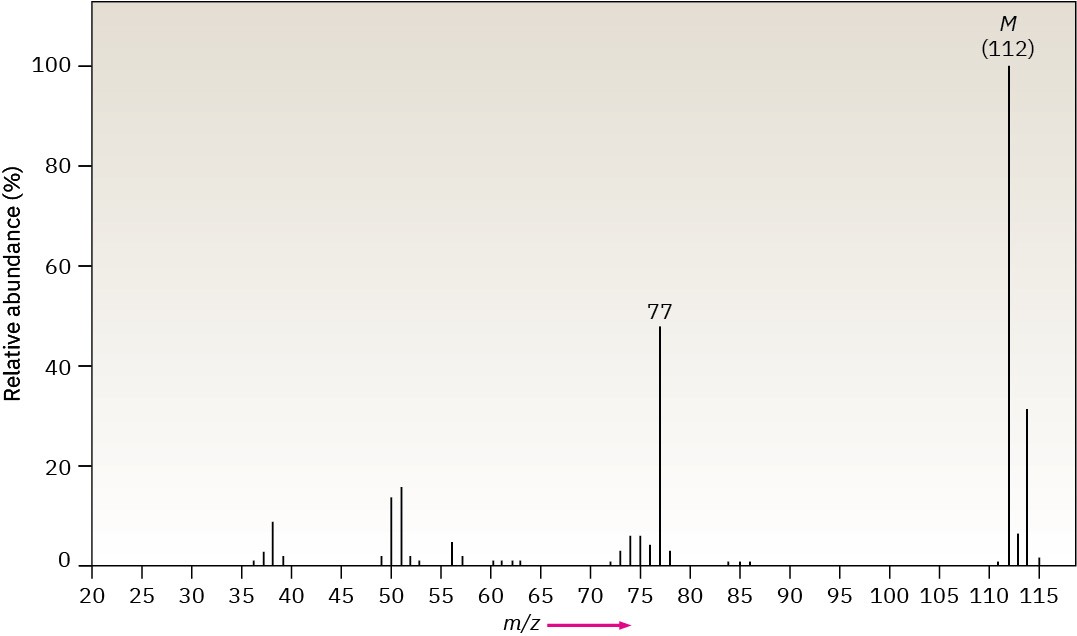
The infrared spectrum of the compound with the following mass spectrum lacks any significant absorption above 3000 cm–1. There is a prominent peak near 1740 cm–1 and another strong peak near 1200 cm–1. Propose a structure.
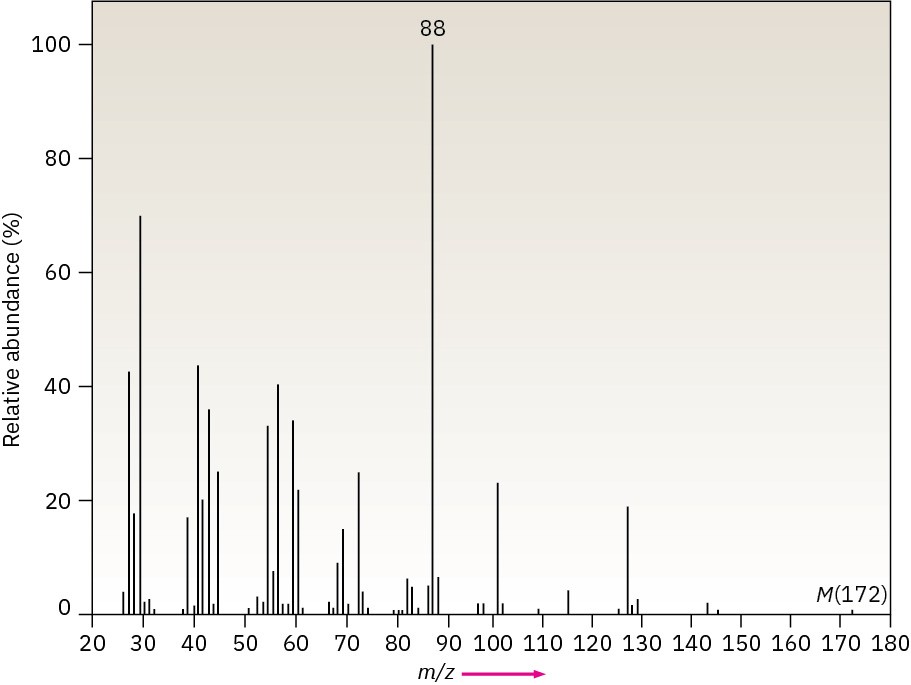
Problem 12-49
The infrared spectrum of the compound with the following mass spectrum has a medium- intensity peak at about 1650 cm–1. There is also a C–H out-of-plane bending peak near 880 cm–1. Propose a structure.
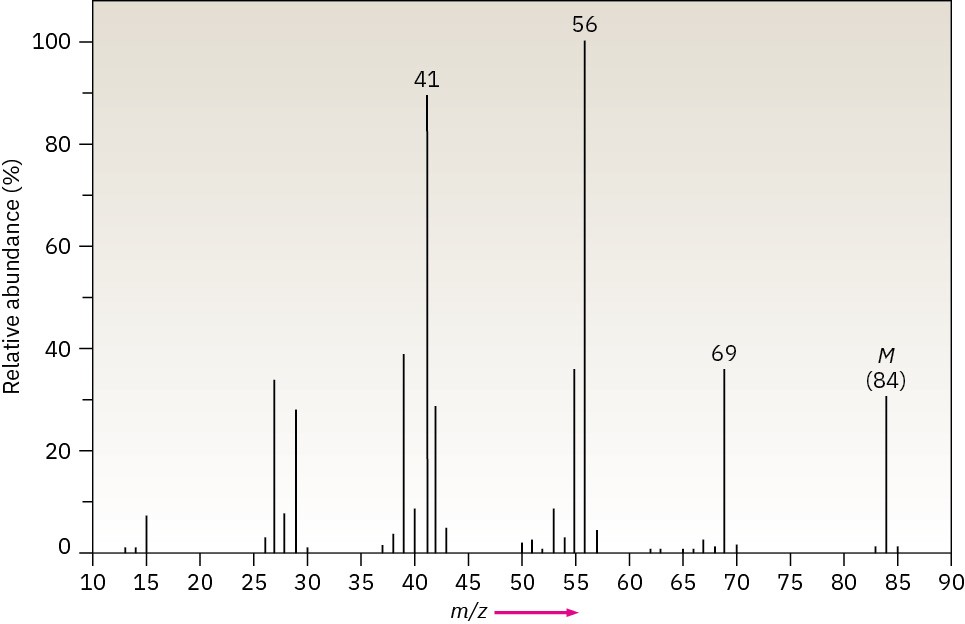
Problem 12-50
The infrared spectrum of the compound with the following mass spectrum has strong absorbances at 1584, 1478, and 1446 cm–1. Propose a structure.