Additional Problems 2
Visualizing Chemistry
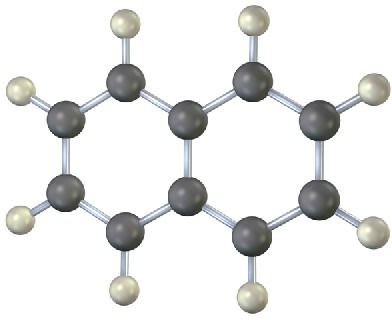
Problem 2-20
Fill in the multiple bonds in the following model of naphthalene, C10H8 (black = C, gray = H). How many resonance structures does naphthalene have? Draw them.
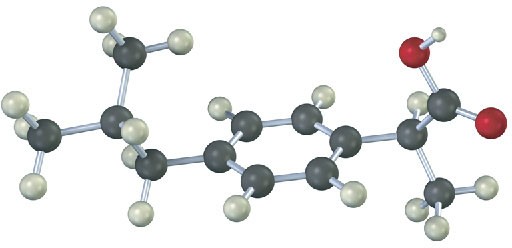
Problem 2-21
The following model is a representation of ibuprofen, a common over-the-counter pain reliever. Indicate the positions of the multiple bonds, and draw a skeletal structure (black = C, red = O, gray = H).
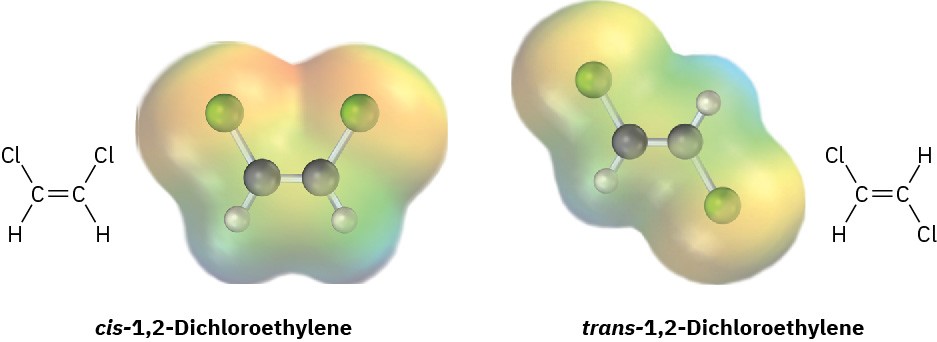
Problem 2-22
cis-1,2-Dichloroethylene and trans-1,2-dichloroethylene are isomers, compounds with the same formula but different chemical structures. Look at the following electrostatic potential maps, and tell whether either compound has a dipole moment.
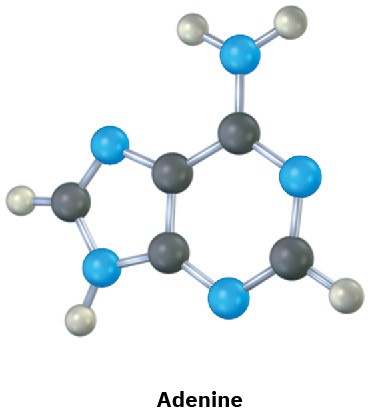
Problem 2-23
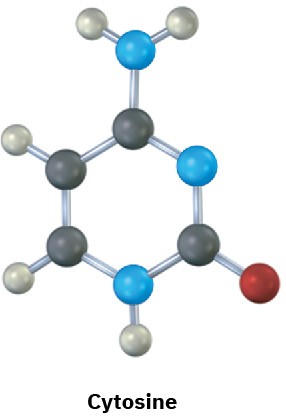
The following molecular models are representations of (a) adenine and (b) cytosine, constituents of DNA (deoxyribonucleic acid). Indicate the positions of multiple bonds and lone pairs for both, and draw skeletal structures (black = C, red = O, blue = N, gray = H).
(a)
(b)
Mechanism Problems
Problem 2-24
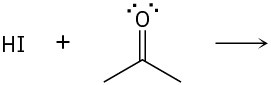
Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds.
(a)
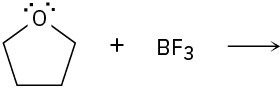
(b)
(c)
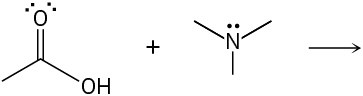
Problem 2-25
Use curved arrows to draw the protonated form of the following Lewis bases.
(a)
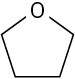
(b)
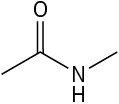
(c)
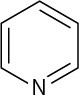
(d)
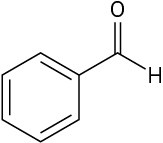
Problem 2-26
Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right.
(a)
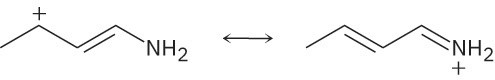
(b)

(c)
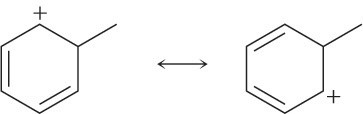
Problem 2-27
Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with HCl and draw the resulting carbocation.
(a)
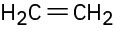
(b)
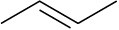
(c)
Electronegativity and Dipole Moments
Problem 2-28
Identify the most electronegative element in each of the following molecules:
(a) CH2FCl
(b) FCH2CH2CH2Br
(c) HOCH2CH2NH2
(d) CH3OCH2Li
Problem 2-29
Use the electronegativity table given in Figure 2.3 to predict which bond in each of the following pairs is more polar, and indicate the direction of bond polarity for each compound.
(a) H3C–Cl or Cl–Cl
(b) H3C–H or H–Cl
(c) HO–CH3 or (CH3)3Si–CH3
(d) H3C–Li or Li–OH Problem 2-30
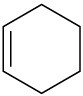
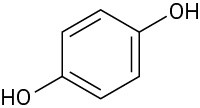
Which of the following molecules has a dipole moment? Indicate the expected direction of each.
(a)
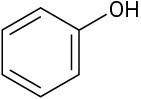
(b)
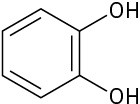
(c)
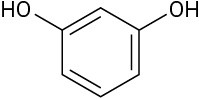
(d)
Problem 2-31 (a)
The H–Cl bond length is 136 pm. What would the dipole moment of HCl be if the molecule were 100% ionic, H+ Cl−?
(b)
The actual dipole moment of HCl is 1.08 D. What is the percent ionic character of the H–Cl bond?
Problem 2-32
Phosgene, Cl2C=O, has a smaller dipole moment than formaldehyde, H2C=O, even though it contains electronegative chlorine atoms in place of hydrogen. Explain.
Problem 2-33
Fluoromethane (CH3F, μ = 1.81 D) has a smaller dipole moment than chloromethane (CH3Cl, μ = 1.87 D) even though fluorine is more electronegative than chlorine. Explain.
Problem 2-34
Methanethiol, CH3SH, has a substantial dipole moment (μ = 1.52) even though carbon and sulfur have identical electronegativities. Explain.
Formal Charges
Problem 2-35
Calculate the formal charges on the atoms shown in red.
(a)
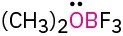
(b)
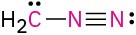
(c)
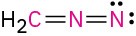
(d)
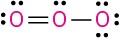
(e)
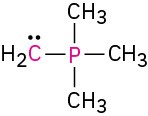
(f)
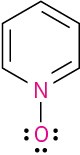
Problem 2-36
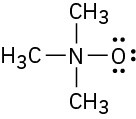 Assign formal charges to the atoms in each of the following molecules: (a)
Assign formal charges to the atoms in each of the following molecules: (a)
(b)
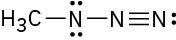
(c)
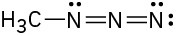
Resonance
Problem 2-37
Which of the following pairs of structures represent resonance forms?
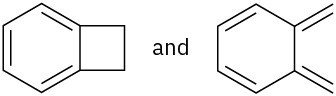
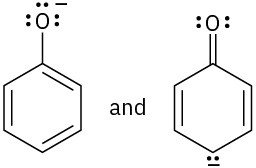 (a)(b)
(a)(b) 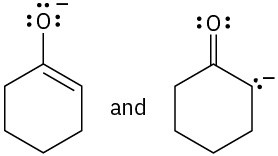 (c)
(c) 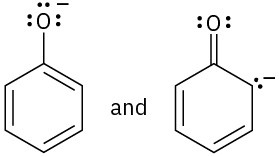
(d)
Problem 2-38
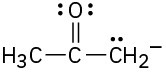 Draw as many resonance structures as you can for the following species: (a)
Draw as many resonance structures as you can for the following species: (a)
(b)
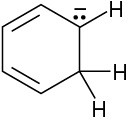
(c)
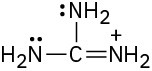
(d)
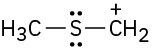
(e)
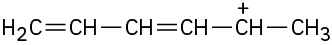
Problem 2-39
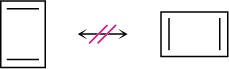
1,3-Cyclobutadiene is a rectangular molecule with two shorter double bonds and two longer single bonds. Why do the following structures not represent resonance forms?
Acids and Bases
Problem 2-40
Alcohols can act either as weak acids or as weak bases, just as water can. Show the reaction of methanol, CH3OH, with a strong acid such as HCl and with a strong base such as Na+ –NH2
Problem 2-41
The O–H hydrogen in acetic acid is more acidic than any of the C–H hydrogens. Explain this result using resonance structures.
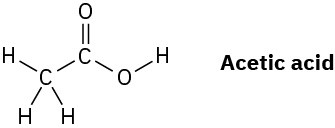
Problem 2-42
Draw electron-dot structures for the following molecules, indicating any unshared electron pairs. Which of the compounds are likely to act as Lewis acids and which as Lewis bases?
(a) AlBr3
(b) CH3CH2NH2
(c) BH3
(d) HF
(e) CH3SCH3
(f) TiCl4
Problem 2-43
Write the products of the following acid–base reactions:
(a) CH3OH + H2SO4 ⇄ ?
(b) CH3OH + NaNH2 ⇄ ?
(c) CH3NH3+ Cl– + NaOH ⇄ ?
Problem 2-44
Rank the following substances in order of increasing acidity:
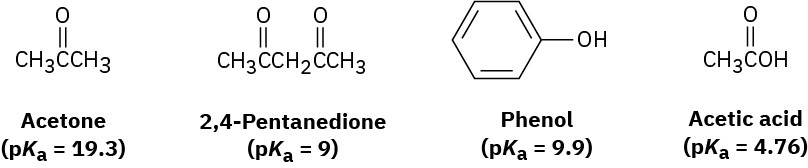
Problem 2-45
Which, if any, of the substances in Problem 2-44 is a strong enough acid to react almost completely with NaOH? (The pKa of H2O is 15.74.)
Problem 2-46
The ammonium ion (NH4+, pKa = 9.25) has a lower pKa than the methylammonium ion (CH3NH3+, pKa = 10.66). Which is the stronger base, ammonia (NH3) or methylamine (CH3NH2)? Explain.
Problem 2-47
Is tert-butoxide anion a strong enough base to react significantly with water? In other words, can a solution of potassium tert-butoxide be prepared in water? The pKa of tert– butyl alcohol is approximately 18.

Problem 2-48
Predict the structure of the product formed in the reaction of the organic base pyridine with the organic acid acetic acid, and use curved arrows to indicate the direction of electron flow.
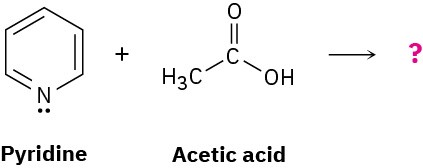
Problem 2-49
Calculate Ka values from the following pKa’s:
(a) Acetone, pKa = 19.3
(b) Formic acid, pKa = 3.75
Problem 2-50
Calculate pKa values from the following Ka’s:
(a) Nitromethane, Ka = 5.0 × 10–11 (b)
Acrylic acid, Ka = 5.6 × 10–5
Problem 2-51
What is the pH of a 0.050 M solution of formic acid, pKa = 3.75?
Problem 2-52
Sodium bicarbonate, NaHCO3, is the sodium salt of carbonic acid (H2CO3), pKa = 6.37. Which of the substances shown in Problem 2-44 will react significantly with sodium bicarbonate?
General Problems
Problem 2-53
Maleic acid has a dipole moment, but the closely related fumaric acid, a substance involved in the citric acid cycle by which food molecules are metabolized, does not. Explain.
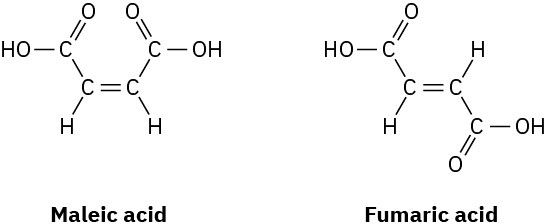
Problem 2-54
Assume that you have two unlabeled bottles, one of which contains phenol (pKa = 9.9) and one of which contains acetic acid (pKa = 4.76). In light of your answer to Problem 2-52, suggest a simple way to determine what is in each bottle.
Problem 2-55
Identify the acids and bases in the following reactions:
(a)
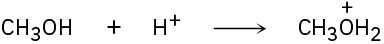
(b)
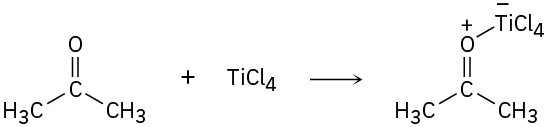
(c)
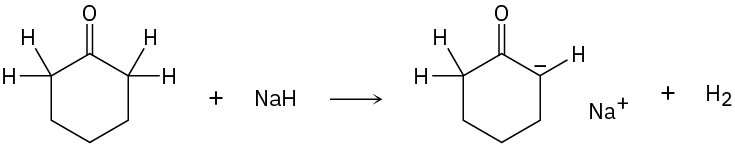
(d)
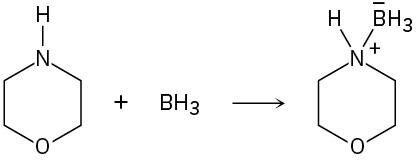
Problem 2-56
Which of the following pairs represent resonance structures?
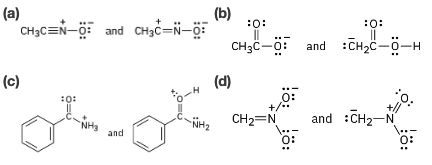
Problem 2-57
Draw as many resonance structures as you can for the following species, adding appropriate formal charges to each:
(a)
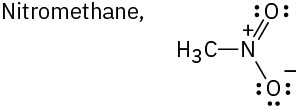
(b)
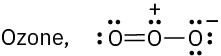
(c)
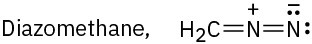
Problem 2-58
Carbocations, which contain a trivalent, positively charged carbon atom, react with water to give alcohols:
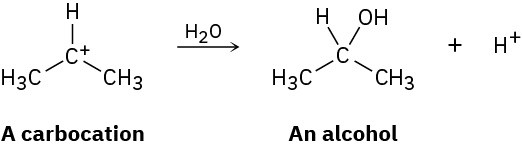
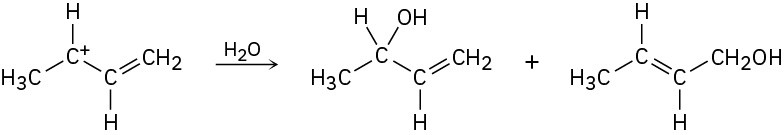
How can you account for the fact that the following carbocation gives a mixture of two
alcohols on reaction with water?
Problem 2-59
We’ll see in the next chapter that organic molecules can be classified according to the functional groups they contain, where a functional group is a collection of atoms with a characteristic chemical reactivity. Use the electronegativity values given in Figure 2.3 to predict the direction of polarization of the following functional groups.
(a)
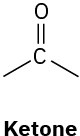
(b)
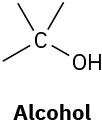
(c)
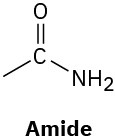
(d)

Problem 2-60
The azide functional group, which occurs in azidobenzene, contains three adjacent nitrogen atoms. One resonance structure for azidobenzene is shown. Draw three additional resonance structures, and assign appropriate formal charges to the atoms in all four.
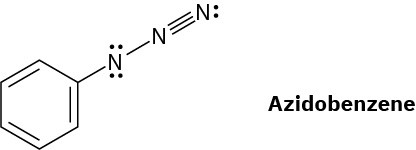
Problem 2-61
Phenol, C6H5OH, is a stronger acid than methanol, CH3OH, even though both contain an O–H bond. Draw the structures of the anions resulting from loss of H+ from phenol and methanol, and use resonance structures to explain the difference in acidity.
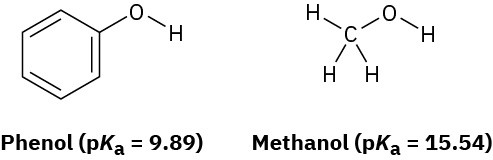
Problem 2-62
Thiamin diphosphate (TPP), a derivative of vitamin B1 required for glucose metabolism, is a weak acid that can be deprotonated by a base. Assign formal charges to the appropriate atoms in both TPP and its deprotonation product.
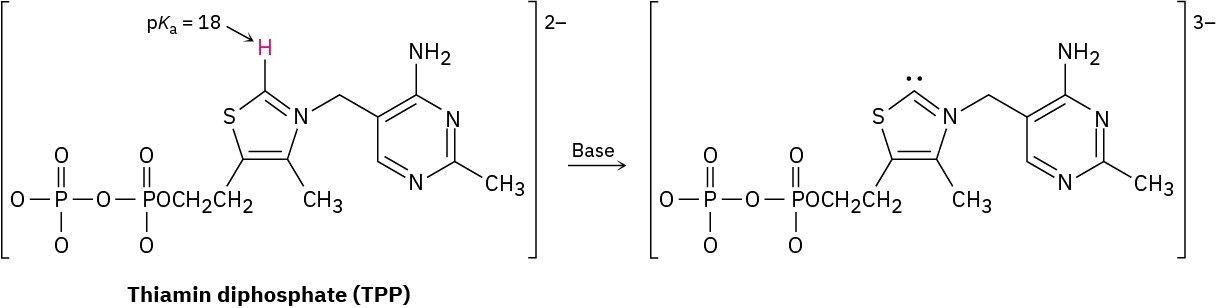
Problem 2-63
Which of the following compounds or ions have a dipole moment?
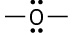 (a) Carbonate ion (CO32–)(b)(c)
(a) Carbonate ion (CO32–)(b)(c) 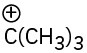
Problem 2-64
Use the pKa table in Appendix B to determine in which direction the equilibrium is favored.
(a)
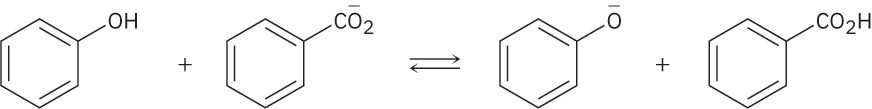
(b)

(c)

Problem 2-65
Which intermolecular force is predominantly responsible for each observation below?
(a)
CH3(CH2)29CH3, a component found in paraffin wax, is a solid at room temperature while CH3(CH2)6CH3 is a liquid.
(b)
CH3CH2CH2OH has a higher boiling point than CH4.
(c)
CH3CO2H, which is found in vinegar, will dissolve in water but not in oil. Assume that oil is CH3(CH2)4CH3.

